On a day in October 2006, I sat in a dark laboratory at Yale University and zoomed in on the fossilized ink of a 200-million-year-old squid relative under an electron microscope. An ocean of translucent balls, each roughly a fifth of a micron in diameter, loomed into view. To the untrained eye, they might have been unimpressive. But I was riveted. These ancient structures looked exactly like the granules of melanin pigment that color the ink of modern squid and octopuses.
Perhaps I should not have been so surprised at the resemblance. Researchers had announced the first discovery of fossil ink granules a couple of years earlier. But seeing them with my own eyes was a revelation. As I examined cephalopod specimens from various locales and time periods, I realized their ink was always the same, perfectly preserved for hundreds of millions of years.
The consistently superb preservation of the ink made me wonder whether melanin might persist in fossils of other kinds of organisms. Melanin is the same pigment found in hair, skin, feathers and eyes. It can impart red, brown, gray and black hues and create metallic sheens. If I could find melanin in other fossils, perhaps I could reconstruct the coloring of extinct animals, including dinosaurs. For decades scientists assumed that pigments hardly ever survive the fossilization process. The few known examples all came from fossils of invertebrate creatures, not backboned ones. Thus, researchers could only guess at the colors of most long-vanished animals, using modern ones as a guide. As a result, dinosaur reconstructions have varied widely: some sport the drab earth tones associated with reptiles and amphibians; others flaunt the rainbow hues of modern birds (the only dinosaurs that have survived to modern times).
But discoveries I and others have made since the mid-2000s are taking out some of the guesswork. Our examinations of dozens of fossils have revealed many examples of melanin-bearing structures. By studying the shapes and organizations of these structures, we have been able to deduce the actual colors and patterns of extinct dinosaurs and other animals from deep time. These clues to the physical appearances of the creatures, in turn, have led to intriguing insights into their behaviors and habitats.
To test my hypothesis that melanin survives in other fossils and can be used to deduce the true colors of extinct animals, I wanted to find and analyze fossils with dark stains indicative of organic preservation in those anatomical regions generally known to contain melanin: the outer covering of the body and the eyes. And I needed to be able to examine the darkened areas under the electron microscope, which might require cutting a specimen down to size. Well-preserved fossils are rare, however, and museums guard them closely. Fortunately, a remarkable fossil site in my home country of Denmark called “Fur and Ølst Formation” had yielded exquisite bird fossils with feathers, which would be an ideal test case. I managed to convince the curator of vertebrate fossils at the Geological Museum in Copenhagen to cut down a typewriter-size block of limestone containing a skull of a little bird with stains where the eyes used to be and a dark halo of feather impressions into a piece the size of a slice of bread so that it could fit into the museum's electron microscope.
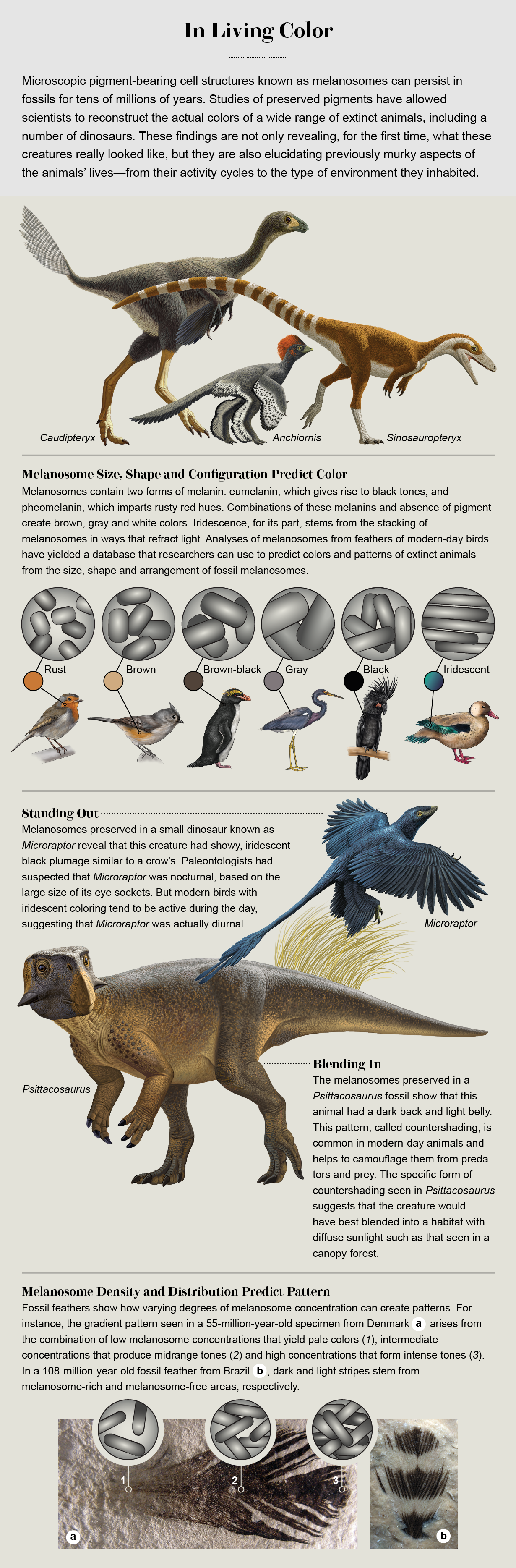
I had a good idea of what to search for under the microscope. Before obtaining the fossil bird for analysis, I had read numerous scientific papers to figure out what melanin looks like in the feathers of living birds. Melanin is synthesized in specialized cells known as melanocytes by cellular components called melanosomes. Typically the melanin remains encased in the melanosomes, which measure about 0.5 to two microns long and take two forms: a sausage-shaped kind that produces a form of melanin called eumelanin, which absorbs all wavelengths of light and thus gives squid ink and raven feathers their black color, and a meatball-shaped variant that makes pheomelanin, which imparts a rusty red hue. An absence of pigments results in white plumage. Gray and brown colors, for their parts, appear to arise from combinations of eumelanin, pheomelanin and pigment absence.
I had also consulted one of the world's leading experts on bird color: Richard Prum of Yale. Because I knew from the fossil ink that eumelanin could be preserved, I figured I would start by looking for that pigment in the feathers. Talking to Prum and his then Ph.D. student Vinod Saranathan, I learned that the sausage-shaped melanosomes line up in a distinctive way along the barbs and barbules that constitute a feather's branches. The melanosomes arrive there during development, when the melanocytes transfer them into specialized cells called keratinocytes that give rise to feathers and hair. If the dark stains on the feather impressions evident in the Danish bird fossil came from melanin, then I should see the sausages arranged this way along the feather branches under the microscope.
With great anticipation, I zoomed in on the fossil feathers—and encountered millions of sausage-shaped structures. Unfortunately, the local underground railway was less than 50 meters from the museum's basement, where the electron microscope was located; vibrations from the constant train traffic made it impossible to get a clear image. But the images were good enough to see the sausages. I immediately e-mailed them to my then Ph.D. supervisor at Yale, Derek Briggs, a pioneer in the study of extraordinarily preserved fossils. He replied with less enthusiasm than I had hoped for, noting that these structures were the same as those he and others had found in fossil feathers and mammal hair for decades and had identified as bacteria.
I still thought the sausages were melanosomes, though, and made my argument to Briggs. Not only did they have the right shape and size but their orientation in the feather structures mirrored that of black melanosomes in modern bird feathers. Furthermore, it was clear from the fossil squid ink that melanin can be fossilized. Briggs began to warm to the idea, but he was not convinced until he showed the images to Prum, who confirmed that they resembled melanosomes in every aspect.
To bolster the hypothesis that melanosomes can persist in fossils of extinct birds, Briggs wanted to find another example. He rummaged through the scientific literature for a good test case and found a description of a little Cretaceous feather from Brazil with preserved, distinct black and white color bands. Briggs thought that if we could show that this specimen also had preserved aligned melanosomes—but only in the dark bands because white coloration stems from a lack of pigment—we would have enough evidence to make our case. We managed to get the specimen on loan and put the entire block under the electron microscope. Lo and behold, when I examined the dark bands of this 108-million-year-old feather, thousands of little melanosomes aligned along the axes of the fine feather branches came into focus. When I looked at the white bands, in contrast, I saw nothing but rock matrix—which is exactly what one should expect in the absence of pigment.
Paint by Number
Since the publication of our melanosome discoveries in 2008, my team and several others have described melanosomes and other pigments from additional fossils. Researchers have also investigated the chemistry of fossil melanin and substantiated our observations that melanin can survive for millions of years, almost chemically intact. Together with Caitlin Colleary, then a master's student at the University of Bristol in England, where I now work, we showed that the slight alterations evident in the fossil melanin are the result of sustained exposure to elevated pressure and heat in the ground. (A few investigators still maintain that the observed structures might be bacteria, but they are running out of options to support their claims.)
Some of our most spectacular findings have uncovered the colors of dinosaur feathers. In 2009 my Yale colleagues and I teamed up with Matthew Shawkey and Liliana D'Alba, both now at Ghent University in Belgium, and others to reconstruct the color pattern of Anchiornis huxleyi, a small, predatory, feathered dinosaur from China that lived around 155 million years ago. Like the Danish bird I had studied previously, the Anchiornis fossil had some dark stains visible to the naked eye, indicating the presence of organic material, probably melanin. But because we were aiming to reconstruct the pattern of its full plumage—a much more ambitious task than simply determining the presence or absence of melanosomes—we could not rely on these stains to tell us all we wanted to know. Instead we had to develop a way to objectively predict colors from the shapes of the melanosomes. To do this, we studied melanosomes from 12 black, 12 brown and 12 gray feathers of modern-day birds. By considering the length, width and aspect ratio of the melanosomes, as well as how much they vary in shape, we could predict feather color using a statistical method called quadratic discriminant analysis with 90 percent accuracy.

When we applied our method to the melanosomes of Anchiornis, the results were striking. Our statistical predictions indicated that the feathers that covered much of the creature's body were mostly gray. The long feathers on the animal's arms and legs, in contrast, were unpigmented by melanosomes and thus white, except for the melanosome-laden tips, which we predicted were black. (Modern birds often have black-tipped wing feathers. The melanin, in addition to coloring the feathers, also fortifies them against battering winds. Perhaps Anchiornis benefited from this strengthening property of melanin, too.) Most surprising, the feathers on the crown of the head contained impressions of round melanosomes—the “meatballs”—that would have given Anchiornis a ruddy crest. All told, this combination of colors made for a spectacularly flamboyant creature.
At around the same time we published our Anchiornis study, Fucheng Zhang, then at the Institute of Vertebrate Paleontology and Paleoanthropology in Beijing, Michael J. Benton of the University of Bristol and their colleagues reported that they had found fossil melanosomes in a range of birds and dinosaurs recovered from 130-million-year-old rocks in China. The pattern of meatball melanosomes in one fuzz-covered dinosaur, Sinosauropteryx, implied that it had sported a reddish coat and a tiger-striped tail, making it the first known ginger dinosaur.
Since those early days our feather data set has grown to comprise hundreds of samples, including ones that allow us to accurately predict iridescence, the metallic sheen seen in the plumage of hummingbirds and peacocks, among other birds. Melanosomes responsible for this effect tend to be longer than typical ones, and they may even be hollow or flattened. The iridescence arises from the packing of the melanosomes within the feather. Certain configurations refract light in ways that create different colors, depending on the angle at which the animal is viewed or illuminated.
Amazingly, in 2009 we found evidence of iridescence in a 49-million-year-old fossil feather from Messel, Germany. The fossil, kept at the Senckenberg Museum in Frankfurt, preserves the original arrangement of melanosomes that generated the iridescence. They were packed into a dense, smooth layer found in the finest branches of the feather fossil, the barbules. There the melanosomes occurred strictly on the farthest edge of the feather and on the top surface, the only part that was not obscured by other, overlapping feathers. We deduced that the tips were iridescent because that arrangement of melanosomes is known to produce what is called thin-film interference, the kind that occurs when gasoline floats on water and creates a vivid rainbow of colors.
It was not long before we discovered evidence of iridescence in an actual dinosaur—a crow-size creature from China with wings on all four limbs. Dubbed Microraptor, it was a primitive cousin to Jurassic Park's Velociraptor. The movie depicted Velociraptor with scaly skin, but scientists now know that both these dinosaurs were, in fact, covered in feathers. In Microraptor, the preserved feathers contain long, sausage-shaped melanosomes arranged to bend light in eye-catching ways. Its plumage thus would have been black, with the same shiny sheen as a crow's. Microraptor is not the only extinct creature now known to have had that rainbow shimmer. Jennifer Peteya of Oberlin College and Ghent's Shawkey have described shimmering iridescence in an enantiornithine bird, called Bohaiornis, and a Jurassic theropod with a big, fan-shaped tail, named Caihong.
More Than Skin Deep
Beyond allowing paleontologists and artists to reconstruct extinct organisms more accurately, fossil pigments are revealing previously unknown facets of the daily lives of both dinosaurs and other long-gone creatures. For instance, experts had presumed that Microraptor was nocturnal, based on the large size of its eye sockets. But our discovery that it possessed iridescent plumage suggests otherwise because in modern birds such coloration is typically found in species that are active in the daytime. The bold coloring of Anchiornis, for its part, probably helped to attract mates or served as some other kind of display, as occurs in flashily dressed modern birds. Thus, color patterns may provide a way to test behavioral hypotheses about a species using a different line of evidence than usual.
Fossil pigments in one species can also illuminate aspects of the other species with which it interacted. Among insects, most color patterns evolved not to help the creatures attract mates but rather as a tactic to avoid getting eaten. Their pigments can thus provide clues to their predators. Fossils of insects called lacewings offer a fascinating example. Between 170 million and 150 million years ago certain distinctive color patterns made their evolutionary debut in insects. Perhaps the most dramatic pattern to emerge during this time was the eyespot, a marking that resembles the eye of a different kind of animal and serves to startle predators approaching their prey at speed from a distance. Lacewings are among the first creatures known to have had eyespots. What kind of predator were they defending themselves against? Most color patterns of modern insects have evolved as a defense against birds, which are their main predators nowadays. But the lacewings' eyespots predate the origin of birds as we know them. Their predators were instead most likely a small group of dinosaurs called the paravians, which are known to have lived at the same time as these lacewings and are thought to have given rise to birds. Although the fossil record of paravians themselves has not allowed us to unequivocally pinpoint when flight evolved in this group, the appearance of these eyespots in the lacewings hints that some paravian dinosaurs had taken wing by this point and were exerting birdlike predation pressure on the insects.
Other fossil melanosome discoveries have enabled my collaborators and me to reverse engineer the environment in which extinct organisms lived. Our first foray into this realm of investigation began with a particularly splendid fossil of a small, plant-eating dinosaur called Psittacosaurus, a relative of Triceratops. These dinosaurs' skeletons are quite common in northeastern China and are often complete. This specimen stood out even in that good company, however. A thin film drapes its body—the remains of the skin, including delicate scales. And its tail displays long, filamentous bristles that may be precursors to feathers. Previous discoveries of dinosaur feathers have all come from the mostly carnivorous theropod group of dinosaurs. The bristles on Psittacosaurus, a distantly related member of the plant-eating ceratopsian group, hint that plumage might have been far more widespread among the dinosaurs than previously thought.
When I first encountered the specimen in 2009, a year after we had announced the discovery of melanosomes in fossil birds, I saw right away that it preserved evidence of beautiful color patterns all over the body. The patterns were subtle, with fine veining, dots and stripes. And I could see that the animal had a dark back that gave way to a pale belly. That kind of dark-to-light color gradient from back to belly counteracts the light-to-dark gradient created by illumination from the sun. This pattern, known as countershading, is common among modern animals ranging from dolphins to deer, helping both predators and prey blend in with their surroundings and thereby elude detection.
I eventually showed the Psittacosaurus pattern to Innes Cuthill, who is part of a group that studies camouflage at the University of Bristol. It was then that we realized we had the opportunity not only to study countershading in a dinosaur but also to deduce from the fossil alone what kind of environment the creature lived in. To reconstruct an animal's habitat, scientists usually gather clues from fossils of other animals and plants found nearby. This kind of approach is problematic, however, because oftentimes the site where a fossil is discovered is not where the organism lived. The Chinese psittacosaur, for example, was recovered from sediments of an ancient lake. The creature was clearly not aquatic, so its remains must have been transported to the lake from the surrounding terrestrial environment, perhaps by moving water. Our study might be able to provide clues about that setting—specifically, the light conditions under which this dinosaur evolved its camouflage.
Cuthill and his collaborators had recently studied countershading in modern ungulates, the group that includes horses, antelope, camels, pigs and rhinoceroses. Although countershading by definition involves darker coloration on the back and lighter coloration on the underside (except in some animals, such as caterpillars, that live their lives upside down), the intensity of those shades and the nature of the transition from dark to pale differ from species to species. Cuthill's team wanted to investigate how well that variation correlates to variation in the lighting conditions found in different environments. Because sunlight varies depending on the latitude at which an animal lives, as well as the density of vegetation in its habitat, the researchers had theorized that ungulate countershading, too, should differ according to latitude and habitat. Their findings bore out that notion. Broadly speaking, if an animal lives in open habitats, the direct sunlight will create a shadow high on the body, with a very sharp transition to the illuminated areas. These animals usually exhibit countershading that matches this pattern, with dark backs that almost immediately give way to pale bellies and little intermediate coloration in between. Pronghorn antelope offer a great example of this kind of countershading. In closed habitats, in contrast, the diffuse light that filters down through the vegetation scatters in all angles, producing a shadow that hangs farther down the body and transitions to the illuminated area gradually. White- and black-tailed deer, common in North American forestlands, exhibit this pattern.
We knew from our visual inspection of the Psittacosaurus fossil that it had countershading of some sort. We therefore carefully projected the pigment pattern onto an accurate, life-size model of the dinosaur, which we accomplished by enlisting the help of British paleoartist Bob Nicholls. Through this work we determined that the transition from dark to light occurred low on the belly and tail in Psittacosaurus.
To test the function of the dinosaur's color pattern, we painted a second copy of the full-scale model gray. Next we photographed this model in a range of daylight conditions, from gloriously sunny to oppressively cloudy, as well as in open land and underneath conifer trees to capture the shadows cast on it. Next we inverted the dark and light shades in the photographs, effectively creating the ideal countershading patterns for concealing the animal in each of the lighting conditions. By comparing our reconstruction of the actual countershading pattern of the Psittacosaurus with the idealized countershading patterns, we determined that the animal's coloring would have best camouflaged it in a habitat with diffuse light, such as that seen in a canopy forest.
Our work on Psittacosaurus didn't end there. In 2021 we reconstructed its cloaca—the multipurpose orifice for defecation and breeding—in great detail. We could show that while the rest of Psittacosaurus's body was camouflaged, the flaring lips defining the cloacal opening were heavily pigmented and must have been used for signaling of some sort, most likely for mating.
Subsequent studies on countershading found that the tiger-striped Sinosauropteryx was adapted to live in open environments with bright sunshine from above and that it carried a striped tail and a bandit mask of pigmented feathers over its eyes. Psittacosaurus and Sinosauropteryx are found in the same fossil beds, but their countershading tells us that they came from different environments.
A nearly six-meter-long ankylosaur, Borealopelta, was found in the approximately 112-million-year-old marine oil sand beds in northern Alberta, Canada. It was colored with reddish-brown pheomelanin and was countershaded. Living land animals of such size are not countershaded, because there are no predators big enough to threaten them. In other words, for such a big creature to maintain its countershading from generation to generation, the Cretaceous predators must have been vicious enough to have threatened it. Perhaps that doesn't come as a big surprise considering the spine-chilling Herculean dimensions of the theropod predators back then. But now we have rock-solid proof in fossil color pattern of their gruesomeness.
A Vivid Future
Scientists still have much to learn about paleocolor. Our ability to see broad categories of color in fossils—those that stem from the shape and arrangement of melanosomes—is already a massive leap forward from what we knew about ancient hues 15 years ago. But there are other pigments to look for in fossils, including carotenoids, which produce bright reds and yellows, and porphyrins, which produce such hues as green, red and blue. These pigments have turned up in the fossil record on occasion. Researchers have identified carotenoid pigments derived from fossil bacteria dating back several billion years; porphyrins are preserved in a blood-engorged mosquito from 46 million years ago and in the eggs of a 66-million-year-old dinosaur known as an oviraptorosaur. Pigments not known from modern organisms have come to light, too, including some from fossil sea lilies and algae dating to between 300 million and 150 million years ago.
We will probably encounter limitations to the detail with which we can reconstruct paleocolors; over millions of years some information is bound to be lost forever. In addition, because exceptional fossils with organic preservation are rare and precious, we must restrict destructive chemical sampling of them. As techniques advance, however, the new discoveries they afford will undoubtedly change our understanding of the past faster than ever before. Each one will bring us that much closer to seeing dinosaurs and other prehistoric creatures as they really were, in full Technicolor glory.