This is how memory loss begins, Sophie tells me: You show up at work, forgetting that you are supposed to be at a breakfast meeting with a client. You blank on the names of your neighbors. Soon enough you walk into a room without any clue as to why you are there. Sophie, a lawyer in her early 50s, who asked to go by a pseudonym, had been suffering from frequent hot flashes and night sweats, both associated with menopause, but the forgetfulness seemed to be in another league. What was happening to her mind?
Lisa Mosconi, director of the Women's Brain Initiative and associate director of the Alzheimer's Prevention Center at Weill Cornell Medical College in New York City, might know. She has analyzed thousands of positron-emission tomography (PET) scans of patients entering menopause and has seen how their brain metabolism changes over time. “In premenopause, your brain energy is high,” Mosconi says, showing me a PET scan of a young woman's brain. It is lit up by many bright red and orange blotches representing high glucose metabolism—a proxy for neuronal activity. In perimenopause, which hits women in their mid- to late 40s, brain glucose metabolism slows by 10 to 15 percent or more, and the scan changes: red and orange spots give way to more yellows and greens, representing less sugar uptake and lower metabolism. “Then, in postmenopause, brain glucose metabolism slows down 20 to 30 percent, sometimes more,” Mosconi says, showing me the final scan. Now, clearly, the greens have gained territory.
Estrogen is the master regulator of metabolism in the youthful female brain, orchestrating everything from glucose transport and uptake to its breakdown for energy. Mosconi's scans are rainbow-colored evidence that decreased levels of the hormone during menopause, which often starts when women are between the ages of 45 and 55, lead to a “bioenergetic brain crisis,” as she describes it. At some point during this seven-plus-year transition period, up to 60 percent of women experience what is known as menopause-related cognitive impairment: bouts of confusion, distractibility and forgetfulness. These memory problems are normal. The generation of synapses requires energy; as estrogen levels and brain glucose metabolism decline, so does the formation of new connections between neurons.
Fortunately, the impairment is temporary: women rebound, their wits intact, as the brain compensates and taps other sources of energy. A 2009 study found that newly postmenopausal women score just as well on cognitive tests as they did before the transition. Decades later, however, roughly a fifth of them will be diagnosed with Alzheimer's disease. Mosconi and others believe that for many of the 3.6 million women living with the disease in the U.S. alone, menopause might have been a tipping point for cognitive decline.
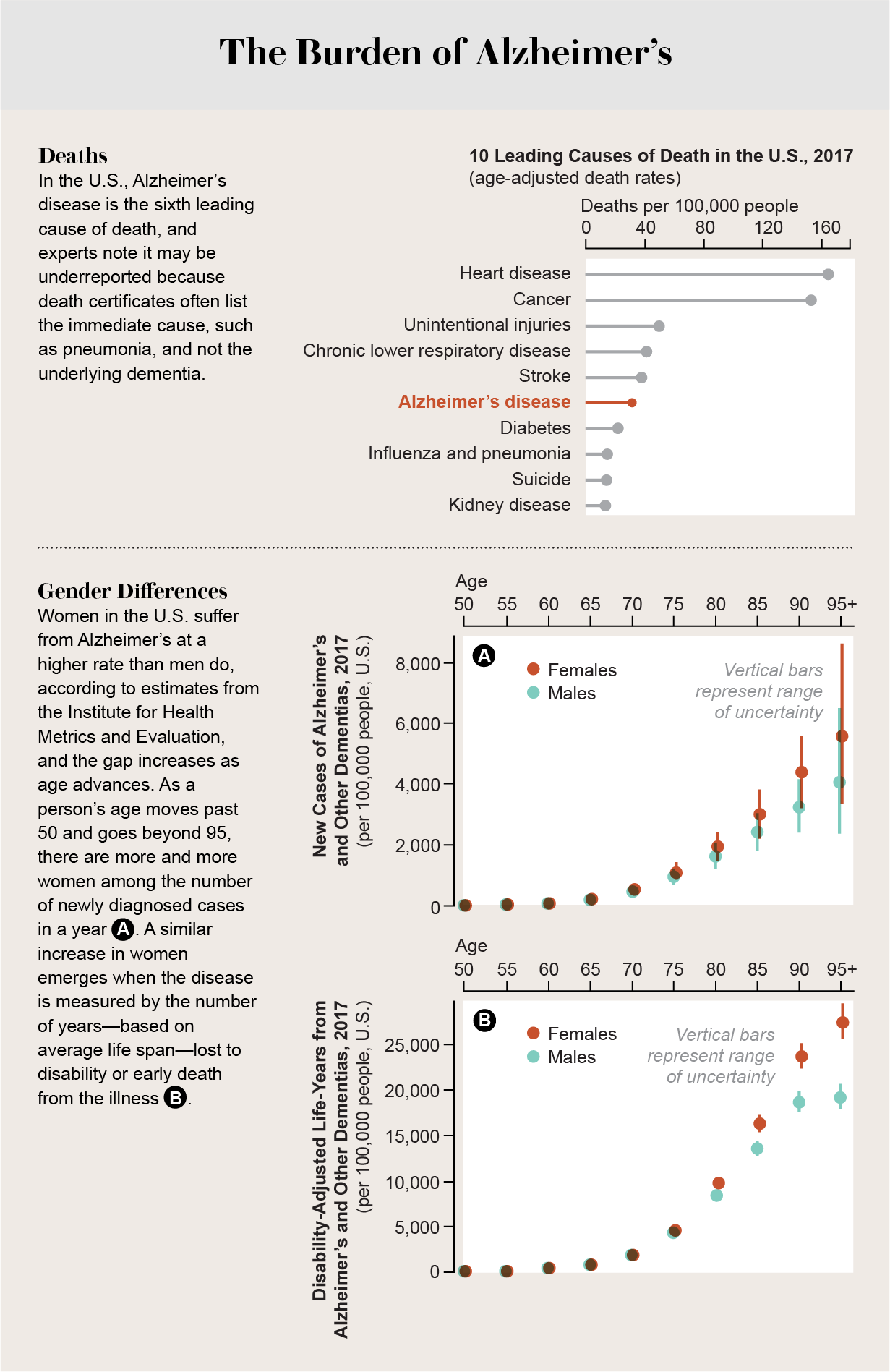
Although investigations of Alzheimer's that focus on women have become a top priority, too many questions remain unanswered when it comes to female-specific risk factors, symptoms, prevention and responses to treatments for the disease. Why in the U.S. does a woman have a one-in-five lifetime chance of developing the disease at age 65, compared with one in nine for a man at the same age? American women live an average of five years longer than men, but “longevity does not wholly explain the higher frequency and lifetime risk,” noted an expert panel representing the Society for Women's Health Research in a 2018 analysis. Why are females who carry the e4 variant of the gene APOE (APOE4), which increases the risk of Alzheimer's, likely to acquire the disease at a younger age than male carriers? What is it about women's biology and life experiences that makes them more vulnerable?
The menopause hypothesis—that decline in estrogen levels in this period renders the brain vulnerable to future damage—could offer answers. If Mosconi and other researchers are right, Sophie and the millions of women worldwide who pass through this transition could benefit from lifestyle interventions and, conceivably but controversially, hormone therapy (HT) to prevent the disease.
Thinking with less estrogen
“It's starvation mode,” says Roberta Diaz Brinton, director of the Center for Innovation in Brain Science at the University of Arizona, describing what happens when estrogen declines and green patches take over in menopausal women's PET scans. Estrogen plays multiple and wide-ranging roles in brain bioenergetics, she explains. A signaling molecule with receptors throughout the brain, it regulates mitochondria, which generate energy for cells and fuel the formation of neuronal connections. Estrogen also activates the enzymes that enable synapses to function, and it facilitates glucose transport from blood vessels into the brain and from the brain into neurons and glia, the cells that support and protect neurons.
Brinton's research on aging female mice has shown that as estrogen levels fall and glucose metabolism slows down, the brain adapts by using ketone bodies—substances produced from fatty acids, in this case from white matter, including the myelin sheaths that protect neurons—as a supplemental fuel source. This switch—essentially an act of self-cannibalization—also appears to occur to some degree in women, and those whose brains draw more heavily on ketone bodies may suffer greater degeneration of white matter and a higher risk of dementia.
Sometimes a brain energy deficit coincides with the development of hard deposits, or plaques, of beta-amyloid protein. They can show up in some brains that function normally, but every person with Alzheimer's has them. They are thought to interfere with synaptic signaling. In brains of those with the disease, beta-amyloid usually comes along with tau, a tangled protein that wraps around the nucleus inside cells, apparently killing them by blocking nutrient transport. Moreover, low estrogen increases the permeability of the blood-brain barrier, potentially exposing the brain to toxins or infections that can stimulate an aggressive immune response, releasing proteins that seed new plaques and tangles.
In contrast to the brains of women in their 40s and 50s, Mosconi says, brains of males in the same age group are not found to have aged significantly, and fewer have beta-amyloid plaques. One explanation is that testosterone, like estrogen, is neuroprotective—and levels of testosterone never drop as steeply or abruptly in andropause as estrogen's do in menopause. This difference might help explain why fewer men get the disease. Alzheimer's pathology may also develop earlier in women than in men, Mosconi explains, but they compensate so well that they are often not diagnosed until the disease has progressed to a later stage. A 2019 study found that women whose PET scans show biomarkers of Alzheimer's outperform their male counterparts on verbal memory tests. If cutoff scores were sex-specific, the disease could be caught earlier, when intervention is more effective.
To further identify women at risk, researchers have begun to investigate connections between Alzheimer's and lifetime exposure to estrogen. Scientists measure estrogen exposure in terms of the “reproductive period”—the time span between a woman's first menstrual period and her last. A large-scale study of 15,754 members of the health care consortium Kaiser Permanente found that women with a 21- to 34-year reproductive period have a 26 percent higher chance of developing dementia than those with a 39- to 44-year period, suggesting that late onset of menstruation or early menopause poses a higher risk. Yet many factors affect women's lifelong estrogen exposure, and their impact is understudied. For instance, a woman's circulating estrogen is dramatically elevated during pregnancy, but after she gives birth it drops and, for several years, remains at a lower level than that in women who have never been pregnant. But studies that sought to link the number of times a woman has given birth to Alzheimer's risk yielded conflicting results. More than 100 million women worldwide take birth-control pills, which suppress ovarian hormones, yet shockingly little is known about their long-term effects on dementia risk.
The Hormone Therapy Dilemma
Sophie, who started taking the pill when she reached puberty and who has never given birth, says her memory loss peaked in her last year of perimenopause. She often experienced more than three hot flashes an hour—a frequency and severity that correlate with increased dysregulation of glucose metabolism in the brain, greater loss of white matter and a potentially elevated risk of dementia later in life. Sophie's doctor prescribed a new pill: a combination estrogen-progestin tablet (progestin protects the uterus). The effect, Sophie says, was “eerily miraculous”: her hot flashes faded, and suddenly she was remembering breakfast meetings again.
It might seem that every menopausal woman should undergo hormone therapy for brain health alone, but the reality is more nuanced. In the early 2000s the National Heart, Lung, and Blood Institute reported results from its massive Women's Health Initiative study and its ancillary memory study showing that HT, usually estrogen plus a progestin, is linked with a heightened risk of breast cancer, stroke, heart disease and blood clots and—in shocking defiance of all expectations—a twofold-higher rate of dementia. Investigators have since identified flaws in the study. Women were prescribed conjugated equine estrogen, a semisynthetic form thought to be less neuroprotective than the 17β-estradiol commonly used today. But a bigger problem was that the women were 65 or older when they started HT.
A woman's age when she takes her first HT pill (or applies her first cream, ring or patch) is central to what Brinton calls the “healthy cell bias of estrogen action.” If neurons are healthy, they are responsive to estrogen. If neurons are aged or deprived of estrogen for too long, they become unresponsive to the hormone because signaling pathways deteriorate and receptors become dysfunctional. In this scenario, adding estrogen might even exacerbate neural degeneration. So for HT to do good and not harm, it must be initiated in the so-called critical window, which is usually within five years of the last menstrual period, Brinton says.
Several observational studies attempted to test the critical-window hypothesis in patients who had taken HT for at least 10 years, and their results run the gamut from a 30 percent reduction in Alzheimer's risk in a Utah-based study in which treatment was initiated within five years of menopause onset to a 9 to 17 percent increase in risk in a recent Finnish study in which age at initiation did not appear to affect risk. Which results should we believe? Researchers do not know. Although they consider HT to be safe and effective for many women at the start of menopause, there remains a lack of consensus about dementia protection that is complicated by a variety of factors. “More clinical trials are needed,” Mosconi says, “especially on women who start hormone therapy while still in perimenopause.” Women with the most severe perimenopausal symptoms, such as Sophie, might be unable to naturally adapt well to the loss of estrogen; in them, HT may prevent neurodegenerative damage during the transition to menopause.
“I don't dare go off it,” Sophie says of her HT. She feels that it saved her from a downward spiral of memory loss that would have left her like her grandmother, a loving, strong-willed woman whom Alzheimer's rendered confused and suspicious. Sophie has not been tested for the APOE4 gene, however, so it is unclear if she would, in fact, have developed the disease—nor have studies confirmed whether HT does help stave it off. Even so, she urges me, a woman in her 40s: “You should start as soon as you need it.” But surely there is a better way to prevent Alzheimer's?
A Window of Vulnerability
Menopause does not cause Alzheimer's. It is more a window of vulnerability—especially for women with underlying risks, Brinton says. At first glance, its connection with Alzheimer's is not obvious. The average age of women at menopause is 51; the average age for a diagnosis of Alzheimer's is 70 to 75. That is a 20-something-year gap. But the so-called prodromal phase—between initial pathology such as beta-amyloid plaques and full-blown cognitive impairment—is also about 20 years. “Maybe the timing is a coincidence,” Brinton observes. “But I don't think so.”
Brain scans aside, is it possible to predict a woman's Alzheimer's risk earlier on, when she is still healthy? In a study published in 2016, Brinton and her colleagues sorted 500 healthy postmenopausal women into three groups: metabolically optimal, borderline high blood pressure and borderline metabolic health. Only one group scored significantly lower on verbal memory tests: women with borderline-unhealthy metabolic health.
Technically these subjects' metabolic measures were still in the normal range. Yet there were clues that their health was going in the wrong direction. For one, blood glucose levels in this group were nearing the threshold of prediabetes, a condition that afflicts about 30 percent of women and is itself linked with cognitive impairment. After a meal the hormone insulin helps glucose enter cells for use as energy, but in someone with prediabetes, cells in the body start to resist insulin. When brain cells become resistant to insulin, they absorb glucose but fail to respond to it—which, compounded by the menopausal slowdown in glucose metabolism, can contribute to neurodegeneration. For many women in this transitional phase, prediabetes is a prelude to type 2 diabetes, which almost doubles Alzheimer's risk. More than 80 percent of Alzheimer's patients are insulin-resistant.
Once we think of menopause—and estrogen depletion—as changing the ecology of the entire body, it is easy to see how a complex array of factors might give rise to Alzheimer's and why managing those factors is key to prevention. Estrogen's healthy effects on the cardiovascular system include cholesterol regulation: it raises levels of “good” HDL (high-density lipoprotein) cholesterol and decreases those of the “bad” LDL (low-density lipoprotein) type that causes the buildup of fatty, waxy deposits in arteries. The APOE gene mediates the metabolism of cholesterol and transports it to neurons; carriers of the e4 gene variant have naturally higher levels of LDL cholesterol in the bloodstream and accompanying hardening of the arteries. Loosened by inflammation, these deposits cause “silent strokes” that more than double the risk of Alzheimer's and other forms of dementia.
Sleep also plays a key role in regulating metabolism, including insulin sensitivity, and deficient sleep affects women disproportionately, especially during menopause. During a normal night of rest, glial cells flush out beta-amyloid and tau proteins. Sleep deprivation disrupts this process, causing the proteins to build up and form plaques, which lead to fragmented sleep, which impairs glucose metabolism, which also interferes with sleep, and so on in perilous loops that accelerate neurodegenerative processes. Again, APOE4 status increases the risk: carriers have a reduced capacity to clear or degrade plaques and tangles.
Stress, too, can move the tipping point during menopause. A 35-year longitudinal study found that the more stressors lasting a month or more women experienced in their 40s and 50s, the likelier they were to have Alzheimer's four decades later. Along with stress, women are more likely than men to report depression, which is associated with a nearly doubled dementia risk. Unsurprisingly, female APOE4 carriers, who, again, have the strongest genetic risk of Alzheimer's, are four times more susceptible than noncarriers to clinical depression, possibly because of increased numbers of beta-amyloid plaques in brain regions involved in emotion regulation.
A window of opportunity
In 2019 Brinton and her colleagues published a follow-up to their study of metabolic indicators, this time with APOE status as a new variable. People with a single copy of the APOE4 gene, which is present in about 25 percent of the overall U.S. population, are more likely to acquire Alzheimer's than others are and represent about 40 percent of all cases. Women develop the disease much earlier than male carriers, between the ages of 65 and 75, possibly because of the loss of estrogen's neuroprotective effects. Carriers have higher LDL cholesterol, more beta-amyloid plaques and tau tangles, reduced hippocampal volume and greater decreases in brain connectivity compared with noncarriers. During the menopausal drop in brain glucose metabolism, female carriers of the e4 allele may rely more on the brain's ketone bodies as an auxiliary fuel.
As in Brinton's previous study, the group at risk for poor metabolic health had lower scores on some cognitive tests. But this time the analysis revealed that APOE4 carriers were the main drivers of the group's poor performance. Among carriers, high cholesterol and other effects of poor metabolic health exacerbated the negative effects of APOE4, leading to early cognitive decline. When carriers in the poorly performing group underwent HT, however, their metabolic health improved, along with their scores on some cognitive tests.
But Brinton sees APOE4 status as “a wake-up call, not a death sentence”: plenty of women with APOE4 do not have the disease. In her study, the group with optimal metabolic health, which had the best scores on cognitive tests, included carriers of the Alzheimer's gene. Were those women, along with healthy noncarriers, better at compensating for the “bioenergetic crisis” of menopause? Did their fitness offset other risk factors?
At least one third of Alzheimer's cases are linked with diabetes, obesity, poor diet, and other factors that are preventable and treatable, according to an oft-cited 2017 report in the Lancet. “The take-home message is that sustaining metabolic health sustains cognitive health,” Brinton concludes. “You can't change your chromosomal sex or age or your gene variant. But you can change your metabolic health and thus your level of risk.” Mosconi agrees. Everyone, especially women in their 40s and 50s, should “know their numbers,” she says, meaning APOE status, metabolic profile, blood biochemistry—even brain scans, especially as new sex-specific imaging biomarkers emerge. “I hope scans will become part of the clinical workup for all middle-aged women (and men) for preventive reasons, just as we have our breasts and uterus checked,” she says. The mantra is “prevention,” a word once seldom paired with Alzheimer's.
Whether HT should be part of a protocol remains controversial. But precision medicine—which uses genetic testing and data analytics—is coming to HT, Brinton says: doctors may soon prescribe precision therapies based on biomarkers of risk such as APOE status, reproductive history, menopausal symptoms, and other factors. And new versions of HT are in the works. Karyn Frick, a neuroscientist at the University of Wisconsin–Milwaukee, and her collaborators have developed a “stripped-down” version of 17β-estradiol that is thought to reduce the risk of breast cancer associated with standard HT. The drug, which has yet to undergo clinical trials, showed promise in preliminary studies in mice. “It acted as a memory enhancer,” Frick says.
For the Alzheimer's cases that cannot be prevented, Brinton's laboratory is developing a treatment called Allo based on allopregnanolone, a naturally occurring steroid that stimulates the production of new neurons. In a mouse model of Alzheimer's, Allo reversed cognitive deficits and restored learning and memory. In a promising phase 1 clinical trial, patients with mild dementia showed regenerated gray matter volume in their hippocampus and a reduction in brain inflammation. Brinton says a phase 2 clinical trial with APOE4 carriers, funded by the National Institute on Aging, is scheduled to begin later in 2020.
In 2016 the National Institutes of Health began to require that the research it funds regard sex as a biological variable. The slow course of Alzheimer's means that years will pass before women can benefit from new studies into the menopause transition. Meanwhile prevention remains essential: recommendations include a plant-centered diet that is low in sugar and in trans fats and saturated fats, physical exercise, stress reduction and a nightly seven hours of beta- and tau-clearing sleep, especially for women in midlife. “Women take care of others; we put ourselves last,” Brinton says. “But we can't keep putting off health.”


