To fight a respiratory infection, the body needs a two-pronged attack. First, it sends immune cells to the scene to destroy the pathogen. Then the defense system must keep those first responders from spiraling out of control. If this attempt at “peacekeeping” fails, a run-of-the-mill fever and cough can escalate to a life-threatening illness—which happened to the tens of thousands of COVID-19 patients who have succumbed to the global pandemic caused by the SARS-CoV-2 virus.
For the most part, macrophages—the large immune cells that consume pathogens—are first responders. In the lungs of mice infected with viral influenza, however, a small subset of these white blood cells does just the opposite: They suppress excess inflammation, researchers report today in the journal Science Immunology. These peacekeeping macrophages also reside in human lungs, suggesting they “might be very important to help COVID-19 patients resist inflammation and maybe survive,” says immunologist Yufang Shi of the First Affiliated Hospital of Soochow University in China. The hospital sent staff and supplies to the nation’s city of Wuhan, but Shi was not involved in the new study.
The research began seven years ago, when Kamal Khanna, an immunologist now at NYU Langone Health, noticed something he found to be stunning. At the time, his lab was studying a similar group of macrophages—not in the lungs but in the spleen, a blood-filtering organ in the lymphatic system. On stained mouse tissue viewed under a microscope, the macrophages formed blue rings around immune-cell-rich areas of the spleen. “They looked like nebulas,” Khanna says.
And these cells were not just visually impressive. When the researchers depleted the macrophages using a clever genetic strategy, the mice died just two days after being infected with small amounts of Listeria bacteria they would normally clear. Another observation was also striking: while other immune cells packed infection-fighting zones in the spleen, this group of macrophages stayed put. “And we thought, This compartmentalization has to be present in [nonimmune] organs as well,” Khanna says. The spleen findings, published in 2017, laid the groundwork for the new analysis in lungs.
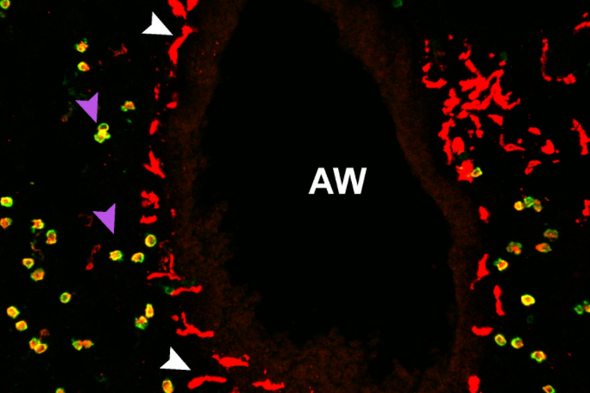
In this complex organ, the vast majority of macrophages live in tiny air sacs called alveoli. But when the researchers examined the lung tissue under the microscope, they saw a much smaller population that was starkly different. Unlike alveolar macrophages (AMs), which are large and round, the rarer macrophages are elongated with sprawling arms — and they’re not found in alveoli. Called nerve- and airway-associated macrophages, or NAMs, these newly identified cells congregate at airways and interact with surrounding nerves. “The whole airway branch gets lit up with these macrophages,” Khanna says.
In another set of studies, his team depleted mice of AMs or NAMs and then infected these animals and normal mice with an influenza virus and compared the level of virus in the groups. These experiments revealed a division of labor: AMs help to fight the virus while NAMs keep the peace and prevent tissue damage.
This kind of differentiation could prove important for designing therapies targeted at inflammation, which is a big problem in COVID-19, says Mallar Bhattacharya, a macrophage biologist at the University of California, San Francisco, who was not involved with the research but calls it a “clever application of novel tools for deletion of specific macrophage subsets.”
NAM-depleted mice produced higher levels of several inflammatory molecules, including one called IL-6 that is involved in so-called “cytokine storms” seen in some patients with severe COVID-19. In a recent study of 191 people treated for the disease in Wuhan, blood IL-6 levels were elevated in patients who died of it, compared with survivors. Clinical trials are now evaluating IL-6–blocking antibodies—drugs that are used to treat rheumatoid arthritis—in COVID-19 patients.
The new study did not address how the intertwining of NAMs with nerves relates to the function of these immune cells. Khanna hopes to gain insight in future mouse studies by depleting NAMs and assessing the health of surrounding nerves or by examining how the airway nerves are affected during different types of infections. The nervous-immune connection is intriguing in light of recent research suggesting that chemical cross talk between gut macrophages and nerve fibers can control peristalsis, the process that moves food through the digestive tract.
A more pressing question is whether NAMs are involved in COVID-19. Toward that end, Khanna is working with NYU Langone Health to obtain fresh lung tissue from people who died of the disease—but doing so is logistically hard and possibly risky. An even bigger challenge for now, in light of New York City’s rising number of cases, is that “basically, our lab is shut down,” Khanna says.
Read more about the coronavirus outbreak here.