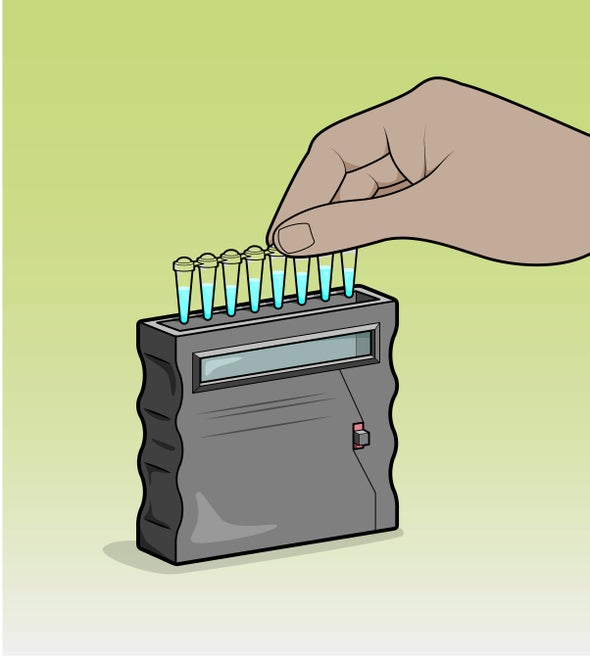
Pollution from industry, agricultural runoff, pharmaceuticals and other sources contaminates water around the world, and detecting it can be expensive and time-consuming. Now researchers have developed a quick, potentially inexpensive way to test for at least 16 dangerous contaminants, including lead, copper and antibiotics, according to a study published in Nature Biotechnology.
The test takes cues from bacteria, which are especially adept at reacting to specific contaminants. “Nature has been solving this problem for billions of years,” says study co-author Julius Lucks, a chemical and biological engineer at Northwestern University. His team searched the literature to find out which proteins bacteria produce to deal with various pollutants. The researchers' new, handheld testing device takes advantage of these proteins' reactions using a series of vials: each has a freeze-dried solution that incorporates a specific protein, which causes the mixture to glow green when an added drop of water contains a particular contaminant.
Each solution includes custom-engineered strands of DNA with one section that a pollutant-sensing protein is bound to and another section that generates a fluorescent glow if activated. The solutions also contain RNA polymerase, which makes RNA by following a DNA strand. If the protein bound to the DNA encounters its corresponding contaminant, the protein changes shape and falls off. This lets the RNA polymerase travel all the way along the DNA strand, making the sample fluoresce green.
The study is “a really nice, clever and creative use of synthetic biology and highlights what the field can do well,” says Mary Dunlop, a synthetic biologist at Boston University, who was not involved in the research.
Researchers have used a similar method to detect pathogens, but this device is the first to identify so many pollutants, Lucks says. The test is “very promising,” says Susan Richardson, a University of South Carolina chemist, who focuses on water issues and was not involved in the research. She cautions, however, that it may need to react to lower contaminant concentrations before it can be widely useful.