In February 1964 Roberto Gilbert Elizalde, a Mayo Clinic–trained surgeon in Guayaquil, Ecuador, found the ideal candidate for a radical procedure being developed in his laboratory. Julio Luna was a 28-year-old sailor who had lost his right hand in a grenade explosion. Gilbert Elizalde, inspired by the successful transplantation of a kidney harvested from a cadaver in the U.S., intended to replace Luna's missing appendage with a donor's.
For nine long hours Gilbert Elizalde and his team worked to prepare Luna's injured limb before skillfully marrying his bones, tendons, blood vessels, muscles, and skin with the forearm of a laborer who had died from a bleeding stomach ulcer. Using recently developed microsurgical techniques, the team stitched together the delicate, tubelike fascicles, nerve-surrounding sheaths that they hoped would guide sprouting sensory and motor nerves from Luna's injured forearm to reinnervate the new hand over the ensuing months.
Exhausted, the team watched nervously as the surgical clamps were released, and Luna's blood perfused his pale new hand to life. Long-distance congratulatory calls circulated. The news made the New York Times: “Dead Man's Hand Is Transplanted.” The hand became one of the first human body parts to be transplanted, after the kidney and cornea. It was a long shot. “Several specialists who were questioned yesterday agreed that the odds against ultimate success were huge,” the Times reported.
For the first week it looked like the skeptics might be proved wrong. When Luna contracted his forearm muscles, tendons in the new hand curled the fingers. Doctors gave Luna an early immunosuppressant, azathioprine, to stop his body from rejecting the foreign appendage. But in the second week it became clear that the immunosuppressant was not enough. When evidence of gangrene appeared, Luna was flown to Boston, where last-ditch efforts to save the hand failed. Twenty-three days after the transplant he became an amputee again.
The medical community both praised and condemned Gilbert Elizalde for this risky surgery. Critics called the procedure unethical, dangerous and unnecessary because it was not needed to save Luna's life—a position on hand transplantation that some experts still hold today. It took another three decades before hand transplantation received a second look.
Over those years surgical techniques evolved, and the development of more effective immunosuppressants (cyclosporine, followed by rapamycin and tacrolimus) allowed transplantation of certain solid organs—kidneys, livers, hearts—to become nearly routine. By the 1990s the success of these powerful pharmacological agents raised hopes of preventing rejection of transplants consisting of multiple tissue types—muscle, skin, bone, nerve and vascular tissue. The field of composite tissue allotransplantation was born. In 1998 a team in France performed the second hand transplant in history, followed shortly thereafter by a group at Louisville's Jewish Hospital in Kentucky. That recipient, Matthew Scott, will soon celebrate the 22nd anniversary of his successful transplant.
Yet hand transplantation remains experimental and, in some circles, controversial. The procedure has been performed only 100 or so times worldwide. Unlike other organ transplants, hand transplantation does not save lives. Recipients undergo a major operation followed by a lengthy recovery and intensive rehabilitation. They face a lifetime regimen of immunosuppressant drugs that can be hard on internal organs and that can increase the risks of certain cancers, infections and other illnesses. Twelve years after receiving his transplant David Savage, whom I will tell you more about soon, lost his life to a cancer that may have been related to immunosuppression.
So why not just use a prosthesis? When I asked transplant recipient Erik Hondusky this question, his answer was simple: “It is a two-handed world.” Hondusky's observation captures feelings expressed by other hand transplant recipients who also shared their dissatisfaction with prosthetics and the strong desire to feel whole again. Prostheses remain insensitive tools; you cannot use them to feel the glance of a spiderweb, or the little bumps marking “F” and “J” on a keyboard, or tiny temperature changes in a cup of coffee. Sadly, Erik developed a staph infection that led to the amputation of his hand nine years after his transplant. He uses a prosthesis reluctantly, only while riding his motorcycle.
Prosthetics come with their own challenges. Despite major advances in technology, a high percentage of amputees choose to give up their upper-extremity prostheses. Our longtime collaborator in Louisville, Christina Kaufman, notes that overall the record of surgical outcomes for hand transplants—and prevention of their rejection—remains impressive, with approximately 80 percent of recipients retaining the hand for at least five years. As techniques for matching immunologically compatible donors and recipients improve, this percentage is expected to grow, along with the number of recipients. Consequently, a successful transplant is no longer simply one that survives rejection. Instead success is increasingly defined based on the extent to which recipients develop functional use of their new hands. And that is where brain science comes into play.
Amputation and the Brain
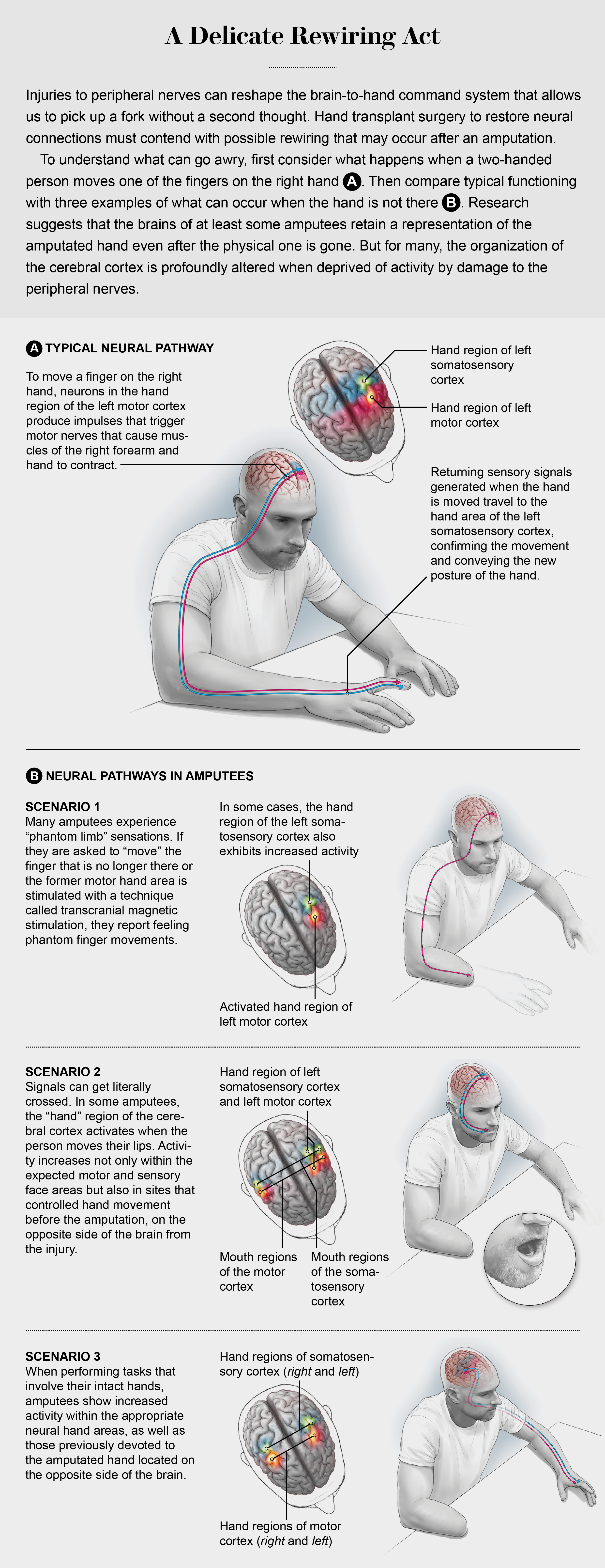
My curiosity about how the brain controls the hands began early, inspired by watching my mother struggle with everyday tasks as a result of her multiple sclerosis, a disease in which one's own immune system ravages the fatty myelin that surrounds neurons in the brain and spinal cord. Her loss of hand function, balance, muscle weakness and spasticity linger as vivid memories and have driven my quest to understand how the brain controls the hands. Our brains dedicate a vast amount of real estate to planning and controlling hand actions. For more than 20 years my lab has been exploring this territory. We investigate the neural mechanisms of hand movements with functional magnetic resonance imaging (fMRI), a technique that allows us to noninvasively assess brain function by tracking local fluctuations in blood flow and oxygenation levels that are coupled to local changes in neural activity.
On a practical level, here is how fMRI works: Imagine that you volunteer for a common (and painfully boring) fMRI experiment that involves alternating the tapping of your fingers interspersed with periods of rest. When moving the fingers on your right side, a population of specialized neurons in the hand region of your left motor cortex (each brain hemisphere controls movements and processes sensations of the opposite side of the body) produces descending impulses, called action potentials. These signals pass through the brain's subcortical structures and down the spinal cord before triggering peripheral motor nerves that cause the appropriate muscles of your right forearm and hand to contract. Specialized receptors in your skin, tendons and joints are stimulated by your finger movements and send feedback signals through peripheral sensory nerves to the spinal cord. There, ascending impulses are relayed via subcortical structures to a specific pool of neurons in the hand area of your left somatosensory cortex, which processes incoming sensory signals.
All of this activity consumes energy. Within fractions of a second tiny capillaries dilate and saturate more active areas of your brain with an excess of oxygen-rich blood (hemoglobin). Changes in local blood oxygen concentrations that accompany neural activity affect the fMRI's magnetic field. Without oxygen bound to it, hemoglobin is strongly attracted to a magnetic field in what is called a paramagnetic state, and oxygenated hemoglobin is weakly repelled (a diamagnetic state). These effects can be captured as a blood-oxygen-level-dependent signal tethered to neural activity. During the little finger-tapping experiment, the hand areas of your left motor and sensory cortices glow with activity on the scanner console.
FMRI can even detect this brain activity in some people whose hands have been amputated. Many amputees experience powerful illusory sensations of a “phantom limb,” the sensation that the amputated appendage is still present. If a researcher asks a person with an amputation to move their phantom fingers, fMRI detects increased activity in the former hand areas. These findings suggest that the brains of at least some amputees retain a representation of the amputated hand even after the physical one is gone—although the story is not quite that simple.
Decades of basic neuroscience research in animals show that the organization of the cerebral cortex changes profoundly when it is deprived of routine activity from a limb—the result of damage to the peripheral nerves. That is, maps of sensory and motor functions in the cortex depend on stimulation. At least in part, the same appears to be true for humans as well. When amputees perform a task with their remaining hand, they exhibit increased activity in sensory and motor cortical areas formerly devoted to the now missing hand. This involvement of the former hand areas occurs in addition to typical activity within those areas dedicated to the healthy hand. Similarly, some brain-imaging studies have shown that movements of the lips may also increase activity in the former hand areas of amputees.
This is where hand transplantation gets very interesting to a brain scientist. Does the mature human brain retain enough plasticity years or even decades after amputation in areas formerly devoted to the amputated hand to take on control of the transplanted hand? The answer to this question could have broad implications for understanding the potential for recovery of function following injuries to the body, spinal cord or even the brain itself.
Brain Recovery
I started exploring this issue when David Savage and his wife, Karen, traveled to my lab, then located at the University of Oregon, a mere four months after his hand transplant surgery at Jewish Hospital in Louisville. If ever there was a case to test the boundaries of post-transplant recovery, David's was it. As a young man, he lost his right hand in a shop accident, and before the transplant he had lived as an amputee for almost 35 years. While we talked, David unzipped the Velcro straps that held his removable splint in place and nonchalantly began opening and closing his new hand. When he saw the stunned look on my face, he cracked a smile, grasped my pen and wrote his name in my notebook. Immediately it became clear who was the professor and who was the student.
Before getting into David's exciting results, we need a short aside to discuss the workings of the peripheral nerves in your hand and arm. Unlike the brain or spinal cord, peripheral nerves are capable of regrowing when injured. They regrow quickly, too—at the astonishingly speedy rate of up to two millimeters per day. A skilled microsurgeon will prepare a patient for this regeneration by carefully segregating the fascicles that encompass the various nerve branches and then delicately suture them to matched fascicles in the donor hand. These fascicles surround vast numbers of microscopic axons—the slender projections growing from the cell bodies of individual neurons—much like conduits surrounding the bundles of multicolored phone wires you might see at a construction site. Once surgically joined, the fascicles guide sprouting motor axons toward hand muscles, where they form neuromuscular junctions. Similarly, axons that send sensory signals to the brain are steered toward the skin, tendons and joints. There sensory nerves produce specialized receptors sensitive to changes in pressure, vibration, and temperature. The process through which peripheral nerves grow back and rejoin the sensory network is called reinnervation.
But even a gifted microsurgeon has limited control over where individual peripheral nerve axons actually terminate in the donor hand. The upshot is that subsequent reinnervation errors present a challenge for recovery of hand function. In David's forearm, the regenerating sensory nerves had inched their way through the repaired fascicles. Along the way, some axons had veered off and innervated patches of skin on his new palm, forming numerous branches capped by tiny sensory receptors. We know this because at this early point in his recovery, David was able to detect and localize light touch along the base of his thumb even though the rest of his hand still lacked sensation. I could not help thinking about how remarkable that was. His brain was receiving input originating in peripheral nerves that had last carried sensory signals from a hand more than three decades ago. These impulses were arising from specialized receptors that had only recently set up camp in an entirely different hand.
Reinnervation error was an issue for David, but his brain still found ways to compensate. A sensory nerve in the forearm that once received input from a patch of skin located, say, on the base of his birth thumb might now carry signals arising from an entirely different location on his transplanted palm. Somehow, in a very short period, David's brain had, nonetheless, learned to interpret the new input it received correctly; if I probed his palm, he experienced the feeling as arising from there and not from his thumb. These perceptions were a few millimeters off but still remarkable considering that until recently David had no right hand for more than three decades. Exactly how the brain solves this puzzle remains unclear. Our working hypothesis is that through the repeated pairing of visual and tactile feedback—seeing and touching at the same time during hand use—brain mechanisms learn to correct for reinnervation error.

As if having waited patiently all this time for the opportunity to again process signals arriving from the hand, the appropriate area of David's sensory cortex responded vigorously when I gently brushed his transplanted palm during an fMRI scan. That is not to say, however, that postamputation reorganization had been fully reversed. As with other amputees, brushing the palm of David's intact left hand also elicited responses in this same area, the right sensory cortex. But he never showed any uncertainty about whether these sensations were coming from his intact or transplanted hand.
David eventually succumbed to cancer, but a transplanted hand can last for decades without any apparent consequences. At more than 21 years postsurgery Matthew Scott—the first case performed in Louisville—has kept his transplanted hand longer than anyone else who has had this operation. He spent 13 years as an amputee after losing his dominant left hand in a fireworks accident that occurred in his 20s. Matt visited us in 2008, nine and a half years after his operation. Feeling had long ago emerged throughout his new hand, indicating that regenerating sensory nerves had completed their journey. He localized touch at all locations on his transplanted hand; on average, he was only a few millimeters less accurate than on his uninjured one. We created a computer-controlled system to stimulate the tips of his fingers during an fMRI session, which revealed distinct maps of each individual digit within the hand area of his sensory cortex.
Although I am tempted to conclude that the organization of Matt's sensory cortex had sprung back to its preamputation organization, this conjecture would be overreaching. We lack data on his brain prior to his amputation, and the fact is that we all have slight differences in the fine-grained organization of our brains, which result from genetics and differing life experiences. We can safely say that Matt's sensory cortex appears to contain a map of his transplanted hand that is within the range of natural variation that we observe in healthy adults. Still, even eight years' post-transplant Matt's brain showed lingering evidence of his amputation. Stimulating his intact right hand also increased activity within the former hand area. How then can his hand function be so good? Part of the answer may involve contributions from other brain regions, located upstream from the hand regions, that are not directly involved in sensing and motor functions.
Simple tasks such as finger tapping or passively experiencing touch are useful means to probe the organization of the motor and sensory cortices. Everyday life, however, requires the ability to grasp and manipulate objects. These more complex, goal-directed actions involve areas of the brain involved with higher-level processing, such as the parietal and premotor areas. These cortical regions use multisensory information about the properties of the object and the positioning of one's body to plan movements targeted to a specific goal, such as grasping a cup to take a drink.
Ken Valyear led a project in our lab that used motion capture and fMRI techniques to study the recovery of visually guided grasping in transplant recipient Donald Rickelman, who had lived as a left-hand amputee for 14 years after losing his hand in an industrial accident. We were particularly interested in the role of the anterior intraparietal cortex (aIPC)—a small region located just behind the sensory hand area that is involved in properly shaping the hand to conform to the perception of objects' shapes, orientations and sizes.
At both 26 and 41 months after receiving his transplant, Donnie, like the other transplant recipients we have studied, showed evidence of persistent reorganization in his motor and sensory hand areas. Not surprisingly, he also experienced impediments in some basic hand functions. Detailed analyses of his hand motions, captured at high resolution as he reached for and grasped objects, revealed substantial improvements in coordination over this same period. How was he compensating for his motor and sensory impairments? To find out, we built a special apparatus that allowed us to ask this question with fMRI. When Donnie grasped objects at 26 months post-transplant, his aIPC and premotor cortex showed weak levels of grasp-related activity relative to people with intact limbs. At 41 months patterns of grasp-related activity had increased within the aIPC and premotor cortex and more closely resembled those of control subjects. We speculate that his improved ability to reach and grasp with his transplanted hand over time may be linked to these higher-level regions picking up the slack for the lagging performance of his reorganized motor and sensory areas.
Donnie and Matt continue to improve their sensory and motor functions many years after receiving their transplants, suggesting that the learning-related changes in the brain may continue to contribute to recovery long after the peripheral nerves have fully regenerated. A major goal of our current work is establishing the relationship between such experience-dependent changes in the brain and use of the hands during real-life activities as measured using wireless wearable sensor technology. These devices allow us to observe at high resolution hand and prosthesis activity over numerous days as participants go about their ordinary lives.
If the superpower of the peripheral nerves is their ability to regenerate when injured, the brain's is its capacity to reconfigure itself in response to changes in stimulation. Both play complementary roles in recovery from bodily injuries. Though in its infancy, work with hand transplant recipients is already showing us that the human brain can respond to the reinstatement of stimulation even after many years of deprivation. These findings challenge fundamental notions about the limits of neuroplasticity in mature adults and may give hope to those struggling to overcome the effects of amputation and other devastating bodily injuries. It may indeed be possible to reinstate the grasping and touch that had been lost decades earlier.