Think about the last time you felt stressed. Did your heart rate quicken? Did your breathing get shallow and fast? Maybe your muscles tensed, and you became more alert? The brain prompts all these physiological changes to help us survive in the face of a potentially life-threatening situation. But when this response is activated inappropriately or persistently, it can become dangerous. Indeed, research has linked uncontrolled stress to a wide range of health problems, from heart disease and diabetes to depression and post-traumatic stress disorder (PTSD).
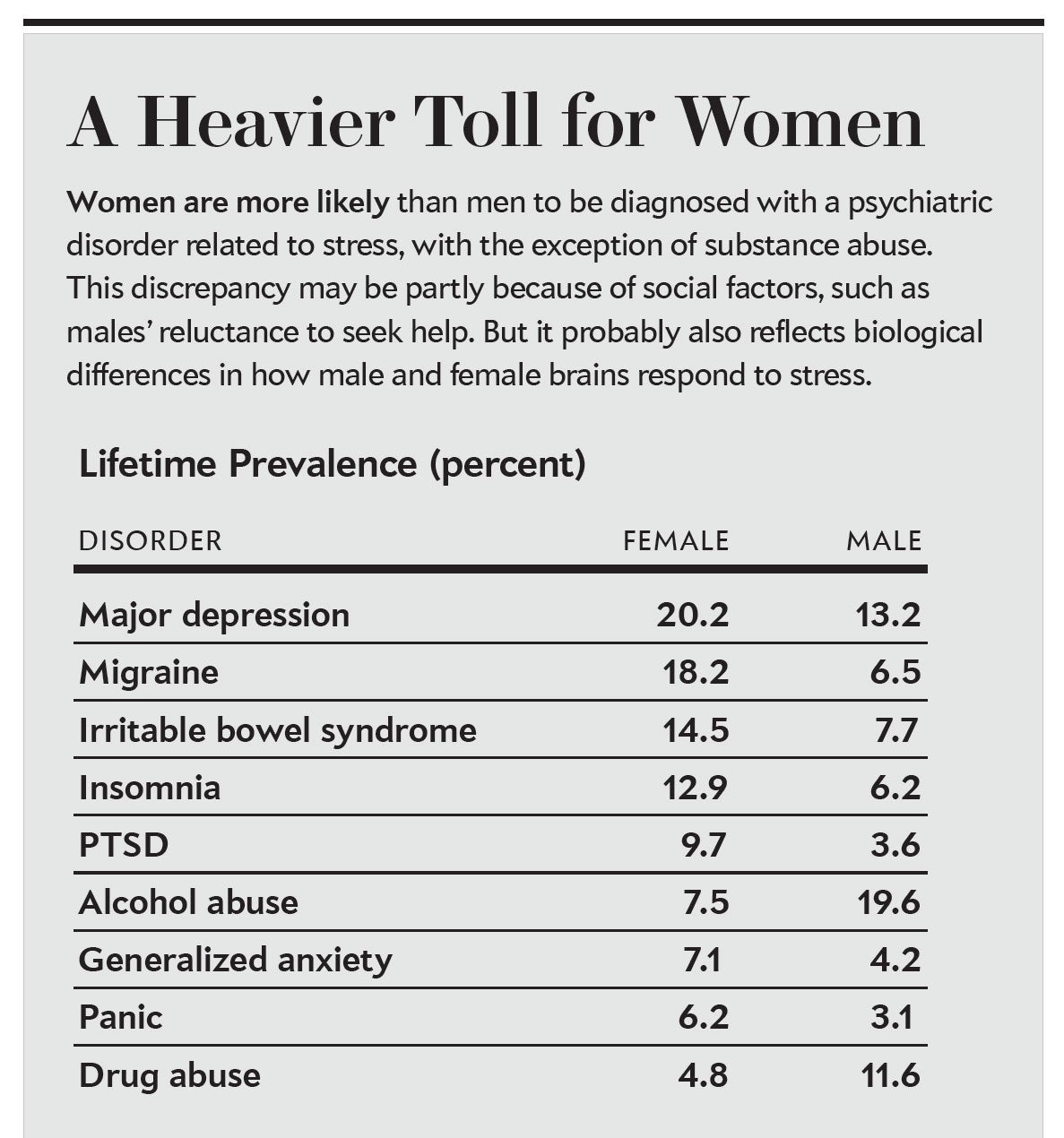
Women are roughly twice as likely as men to suffer from stress-related psychiatric disorders, according to epidemiological analyses. The big question has always been: Why? Some experts argue that cultural factors are at least partly responsible. For instance, women may be more willing than men to seek help for mental illness, making their cases more likely to be counted. But evidence from animal research suggests that biology may also play an important role. Scientists are uncovering telling differences in the ways that male and female brains react and adapt to stress.
This insight has been a long time coming. Historically, scientists studied male animals almost exclusively—even when investigating disorders that seem to occur more frequently in women. One reason is that many researchers feared that fluctuating ovarian hormones would complicate their studies, muddy their data and create a need for more subjects or more time at greater expense. Recent investigations have discredited this line of reasoning—the data collected from female animals are no more variable than those from males—but the male bias in animal research has persisted nonetheless.
To address this issue, the National Institutes of Health launched an effort in 2016 in the U.S. requiring that scientists conducting animal studies include sex as a biological variable in their research by studying both male and female animals. As a result, researchers who study chronic stress stand a much better chance of understanding how it impacts the health of both sexes—work that could lead to more effective, sex-specific treatments for psychological disorders. In fact, some of the most promising new therapies under investigation—including oxytocin for anxiety and ketamine for depression—appear to have very different effects in females than in males.
Female Stress
The animal models scientists use to explore the effects of stress take many forms. Some researchers expose rodents to something stressful—perhaps a brief restraint or a sound they have conditioned the animals to associate with a mild shock—for several days in a row. Others alter levels of stress-related chemicals, such as glucocorticoids or corticotropin-releasing factor (CRF), in the animals’ brains via genetic engineering and other techniques. Regardless of the method, many of these manipulations seem to produce faster and stronger reactions in females. We are just beginning to understand why.
It turns out that the most basic cellular processes involved in the stress response differ between the sexes. For example, neuroscientist Georgia E. Hodes, then working in Scott Russo’s laboratory at the Icahn School of Medicine at Mount Sinai, conducted a study in which she and her colleagues stressed male and female mice over the course of several weeks. They noticed that it took 21 days to increase anxiety and depressionlike behaviors in male mice but only six days to produce the same reaction in female mice. In search of an explanation, they looked to the nucleus accumbens, a brain region involved in seeking out rewarding and pleasurable activities. Disruption of normal brain signaling in this area is thought to contribute to anhedonia, or an inability to experience pleasure, which is a common symptom in depression and various other stress-related disorders.
Within the nucleus accumbens, Hodes identified sex differences in the regulation of a gene referred to as Dnmt3a (DNA methyltransferase 3a). Following a six-day stressor period, it was expressed more in female mice than in males. This gene encodes an enzyme that alters a cell’s DNA in such a way that it prevents other genes from being read and used to make proteins. To determine Dnmt3a’s role in chronic stress, Hodes removed the gene in the nucleus accumbens of female mice. Without it, the females became more resilient and responded more like male mice. These findings suggest that female mice experience an increase in Dnmt3a expression after only a short exposure to stress, which then blocks other proteins that promote stress resilience. Interestingly, researchers are developing drugs that inhibit DNMT enzymes to treat certain cancers. Therefore, similar drugs may be helpful in treating stress-related disorders, particularly in women.
Changes in gene expression are not the only sex differences observed in the brain. When I was a postdoctoral fellow, my mentor, neuroscientist Rita J. Valentino of Children’s Hospital of Philadelphia, and I discovered sex differences in receptors that respond to CRF, a hormone that helps to kick-start the body’s biochemical response to stress. Although there are CRF receptors in many brain regions, we focused on the locus coeruleus, a structure responsible for changing our levels of arousal from sleepy to fully awake. During a stressful event, CRF floods the locus coeruleus, where it binds to CRF receptors to keep an animal on high alert. Typically these receptors sit on the outside surface of brain cells, waiting for a CRF signal. As CRF levels rise, however, the receptors migrate from the cell membrane to its interior, effectively going off-line. This process is thought to protect brain cells from becoming overactivated.
We discovered that in male rodents, CRF receptors withdrew inside neurons after exposure to a standard stressor. They also withdrew in male rodents that were genetically modified to overexpress CRF. In female rodents, though, the receptors lingered on the cell membrane, where they could remain responsive to high CRF levels. The results suggest that CRF may increase arousal and alertness more in females than in males. In some situations, this difference may actually be adaptive: it can be a good thing to stay fully switched on during a stressful occurrence. But overactivation of this system can also lead to hyperarousal, a disruptive state that, in humans, contributes to insomnia, impaired concentration and feeling inappropriately “on edge.
Patients with PTSD and depression can exhibit high levels of CRF and these symptoms of hyperarousal. So if similar sex differences are found with human CRF receptors, it could help explain why women are more likely to suffer from PTSD and depression. This variation may be hard to demonstrate, however. When human brains are being imaged, technical limitations make it difficult to detect molecular changes such as CRF receptor localization. But we have other reasons to believe that women, like female mice, may have a greater sensitivity to CRF: injecting it into the bloodstream causes a greater rise in stress hormones in women than in men.

The Impact of Hormones
Where do these differences come from? Emerging research points to the different complement of genes males and females are born with—as well as hormonal surges in the womb and during puberty, both of which can permanently alter the developing brain. Additionally, fluctuating levels of testosterone, estrogens and progesterone can modulate brain function in adults. In my laboratory at Temple University, we assessed the role that these circulating hormones play in regulating rodents’ behavioral responses to high levels of CRF by looking at compulsive grooming.
When a rat compulsively licks its own fur, sometimes to the point of causing baldness, it is thought to reflect a state of high anxiety. The compulsive grooming may be a form of self-soothing. We found that when we injected CRF into rats to induce stress, females engaged in more compulsive grooming than males did. Moreover, the amount of grooming changed over the course of a female’s estrous cycle, which is similar to the human menstrual cycle but lasts only four to five days. At the phase of the cycle when ovarian hormones—including estrogens and progesterone—peaked, CRF triggered even more compulsive grooming, suggesting that the hormones somehow amplify CRF’s effects.
Behavioral responses such as grooming draw on many brain regions. So in trying to account for the grooming differences between the male and female rats, Kimberly Wiersielis, then a graduate student in my lab, speculated that CRF might activate different circuits in their brains. To test this idea, she examined brain slices for cFos, a protein expressed only when brain cells are switched on. Then we statistically compared cell-activation patterns. Our analysis revealed that CRF activated diverse brain regions in both sexes, but the patterns were different—and especially so between males and females at the point during estrus when females have the highest levels of estrogens and progesterone.
A Sex-Based Rx
These sex differences are not minor. As we search for better remedies for stress-related psychiatric conditions, it is vital that we take them into consideration. To date, potential therapies are most often screened in male rodents. But the same compounds can have very different effects in females. For example, neuroscientists Brian Trainor and Michael Q. Steinman, then both at the University of California, Davis, tested one putative therapy, oxytocin, in both male and female mice. Because this hormone promotes social bonding in mammals, scientists have speculated that administering it to people in the form of a nose spray might reduce social anxiety and avoidance, as well as improve deficits in processing social cues, a problem that is seen in some people who have stress-related psychiatric disorders. Their group found that intranasal oxytocin did indeed reduce anxiety in male mice, but it made female mice even more anxious under certain conditions. We need to make sure that oxytocin sprays will not cause similar adverse side effects in women.
Ketamine provides another example. This medication, normally used as an anesthetic, blocks the N-methyl-D-aspartate receptor, a protein that can regulate many processes, including aspects of the stress response. It has generated a lot of excitement as a potential therapy for depression because, unlike traditional antidepressants that can take weeks to provide any benefit, low doses of ketamine reduce symptoms fast, sometimes after a single infusion. Unfortunately, high doses of ketamine can induce delirium, hallucinations and an “out of body” experience (which is why it is also a popular recreational drug).
Researchers have been investigating ketamine in male animals to develop more targeted therapies for depression. In 2013, though, neuroscientists Mohamed Kabbaj of the Florida State University College of Medicine and Nicole Carrier, then a graduate student in his lab, also tested ketamine in female rats. They found that it took less ketamine to relieve depressionlike symptoms in the females and that it was not just a matter of differences in body mass. Instead a different biological mechanism seemed to be at work. If these same differences exist in humans, researchers working on new drugs with ketaminelike properties may need to develop separate therapies for men and women. Researchers continue to evaluate ketamine for its long-term safety and effectiveness, and these studies may well reveal that, like female rats, women should receive a lower dose. (In 2019 the Food and Drug Administration approved a ketaminelike drug, called esketamine, for use against treatment-resistant depression.)
We do not know why males and females would have evolved different biological responses to stress. One possibility is that a female animal protecting vulnerable young might be well served by being able to maintain a heightened state of alertness and awareness of her surroundings—as would her babies. That advantage might outweigh the downstream risks associated with increased stress sensitivity, such as greater vulnerability to depression and anxiety disorders. Studies are needed, however, to investigate this idea.
As we commit to including female animals in research, we stand to learn more not only about disorders that are more common in women but also about conditions that are more often diagnosed in men, such as autism and attention-deficit hyperactivity disorder. In this case, looking at brain differences in females might reveal factors that confer greater resilience. Better-targeted treatments for both sexes could follow.
It is becoming increasingly clear that the traditional approach of studying only male animals is flawed. In fact, our past reliance on male animals in drug-development studies may explain, at least in part, why women report more adverse drug reactions than men do. The practice may also have kept drugs that would be highly effective in females from ever making it to market. The good news is that thanks to new efforts that promote the study of both male and female animals, change is underway.


