When Magdalene Quintero was 14 years old, her mouth filled with painful ulcers that made eating and drinking unbearable. Her normally tawny skin flamed crimson red across the bridge of her nose and cheeks. The tips of her fingers burst into open sores, as if she had dipped them in acid. She spiked fevers, developed headaches, lost weight and was always tired.
It took a year of visits to various doctors for Quintero to learn that she had lupus, a life-threatening and chronic autoimmune condition that can cause pain, inflammation and damage to any part of the body. It took another two years for her rheumatologist—the only one at the Indian Health Service serving her community of Jones, Okla.—to find medications in the right doses to bring her disease under control.
Quintero, who is now 25, considers herself lucky. Lupus also struck her younger sister, Isabel Hernandez, and has taken a more terrifying form. It began with constant nosebleeds. By the time Isabel was diagnosed, at age 17, her lungs were hemorrhaging blood. She spent 88 days on a ventilator before relearning how to walk, talk and eat. Today, at age 21, her kidneys are failing. Every day she spends hours having her blood filtered by a dialysis machine, waiting for a transplant.
“It is so heartbreaking,” says Quintero, who recently completed her master’s in biomedical science at Oklahoma State University. She worries whether her future holds a similar fate. “But I also feel thankful for my own health and fortunate that the medications that I’m on are doing their job,” she says.
Lupus has been called “the great imitator” because its symptoms—including fever, fatigue, joint pain, rashes, headaches, memory problems and organ failure—often mimic many other autoimmune conditions. Those symptoms reveal the many ways our bodies can turn against us as a wayward immune system, intended to defend us, attacks our own healthy cells.
Traditionally therapies for lupus and other autoimmune diseases have relied on decades-old blunt-force strategies that essentially bludgeon a badly behaving immune system into submission. But these approaches cause collateral damage that sometimes can be worse than the disease itself. Foremost among these treatments are steroids, medications that have unparalleled ability to dampen the entire immune response but in doing so can leave patients vulnerable to dangerous and even deadly infections.
Recent research is fueling a shift in the way autoimmune diseases are treated, based on a more nuanced approach. Distributed throughout the body, the human immune system is a dizzyingly complex network of many different types of cells, organs, tissues and proteins, which communicate with one another through a wide variety of chemical messages. Modern technology, such as gene assays and molecular engineering, is enabling scientists to target individual parts of this network, identifying new targets for treating autoimmunity with much more precision. Some of these new therapies aim to interfere with autoantibodies, rogue antibodies that take aim at healthy cells. Others work by hobbling key chemical messengers that are passed among immune cells.
But these treatments do not work for all patients, and many diseases prove stubbornly resistant, which is why many people with autoimmune problems are drawn to approaches outside of mainstream medicine. Among scientists, the successes and failures are creating a new appreciation for the precarious balance our body maintains between protection and pathology. Half of the immune system—such as cytotoxic or “killer” T cells and antibody-producing B cells—is designed to fight. The other half—mainly regulatory T cells—is designed to keep the peace. When the former goes rogue and the latter fails to keep it in check, autoimmune disease ensues. “All of us, every one of us, has the potential to develop autoimmunity,” says V. Michael Holers, a rheumatologist at the University of Colorado School of Medicine, “because our immune system develops with this kind of yin and yang.”
Early Warnings
For decades steroids such as prednisone and dexamethasone have been the mainstay of treatment for patients with autoimmune conditions. These potent anti-inflammatory drugs indiscriminately shut down the production of cytokines, the key messengers that rouse our vast army of immune cells to fight a perceived threat. Although steroids can be extremely effective at reducing the signs and symptoms of autoimmunity, they exact a price so high that some people liken it to making a deal with the devil. Many patients are stuck taking these medications, orally or by injection, for much of their lives. And steroids can create a whole host of other problems, such as cataracts, mood swings, weight gain, trouble sleeping, thinning bones, high blood pressure, high blood glucose and increased risk of infection.
Furthering the problem is that by the time most patients with autoimmune diseases seek treatment, a lot of damage is already done. Autoantibodies have been stealthily coursing through the blood and tissues for months or years, leading to ongoing assaults on a targeted organ—pancreas, kidneys, joints, gut, skin, hair follicles, brain, spinal cord. As a result, treatments such as steroids are defensive in nature, focused on keeping the disease from getting worse or damping down flare-ups.
Now there is a big push among physician-scientists to go on the offense, correcting autoimmune imbalances before they produce their most devastating consequences. Researchers hope focusing on early treatment and prevention could lower the burden of autoimmune disease, much as treating people at high risk of cardiovascular disease with blood pressure medications and cholesterol-lowering drugs has reduced the prevalence of heart attacks and strokes. Several trials investigating this idea are underway for type 1 diabetes, rheumatoid arthritis, lupus and multiple sclerosis.
There are already some early successes. In one study, researchers recruited 76 people with a family history of type 1 diabetes, who also had abnormal blood sugar levels and at least two diabetes-related autoantibodies. Half the participants received an experimental drug, a monoclonal antibody called teplizumab that interferes with the immune system assault on insulin-producing beta cells in the pancreas. The other half received a placebo. More than five years later only 50 percent of people treated with a two-week course of the drug had developed the disease, compared with 78 percent of those who received a placebo. The researchers estimate that in those who did develop the disease, early treatment delayed onset by nearly three years.
“That’s a big deal,” says endocrinologist Carla Greenbaum, director of the TrialNet Hub, which coordinates the clinical trial network leading the study and others like it. “This is a disease that affects every moment of every day of your life, so any time without it is clinically important.” By fine-tuning this approach, scientists hope they can extend the disease-free period and perhaps discover ways to block the destruction entirely. And Greenbaum thinks the idea should work for other ailments. “We are really the template for all these other autoimmune diseases that are now also discovering these long preclinical periods,” she says.
Multiple sclerosis (MS) is another autoimmune condition where early detection is crucial. It affects the central nervous system, and a telltale sign is white matter lesions, spots on the brain and spinal cord where the disease has stripped the protective covering off the nerves. Over the past decade researchers have found these abnormalities in hundreds of people with no outward symptoms of MS, who came to their notice only after an unrelated concussion or migraine landed them in a brain-scanning machine. David Hafler, a neurologist at the Yale School of Medicine, believes these cases, known as radiologically isolated syndrome (RIS), could represent the earliest-known stage of MS. He and his colleagues have launched a multicenter study with biotech firm Genentech to treat RIS with ocrelizumab, a drug commonly used to treat later stages of MS. And Hafler would like to find other biological hallmarks that would allow intervention even before the lesions appear. “The ultimate treatment for autoimmunity is to identify [people who are at high risk] and treat them before the disease really begins,” he says.
Sharpening the Target
Drugs such as monoclonal antibodies offer the important advantage of being able to go after specific immune system elements that cause a given disease while—unlike steroids—leaving the rest of the immune system functional. But this new targeted approach comes with its share of difficulties. Although it has expanded the therapeutic options for patients, it has also fizzled or even backfired in some cases, making patients worse. Harmony within the immune system, it turns out, is not easy to achieve.
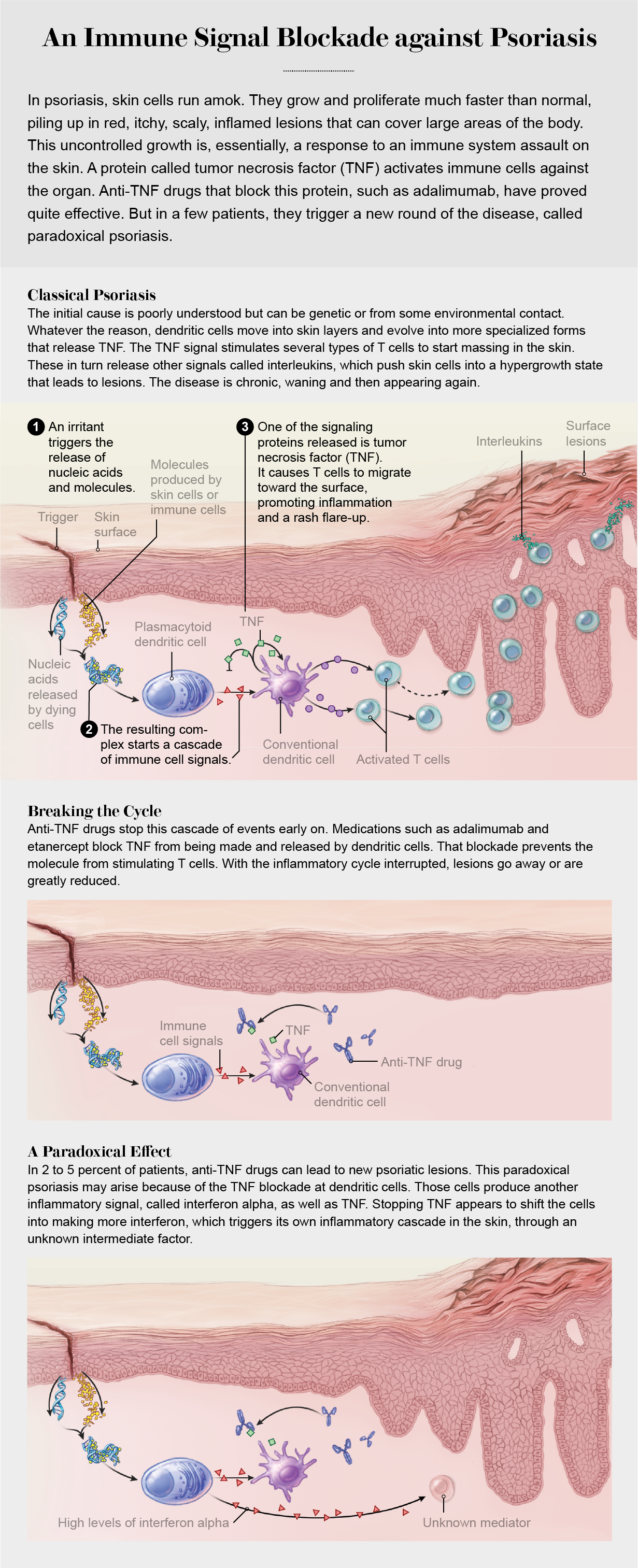
Several targeted therapies focus on a particularly powerful cytokine called tumor necrosis factor, or TNF. It goes haywire in many autoimmune diseases, setting off waves of damaging inflammation. Monoclonal antibody drugs that block its action are widely used in the treatment of rheumatoid arthritis, inflammatory bowel disease and psoriasis. Yet clinical trials in multiple sclerosis showed that TNF inhibitors actually exacerbate the disease. “It is one of the great curiosities of autoimmunity,” Hafler says. What’s more, targeted therapies that suppress autoimmune disease in one patient have been shown to trigger that same disease in other patients.
Belimumab, one of only three new medications approved for lupus in the past 60 years, also is a targeted therapy. It blocks a cytokine called B lymphocyte stimulator, or BLyS (pronounced “bliss”), that keeps autoreactive B cells going, extending an autoimmune reaction. Although the treatment has helped many patients, a significant number of them get no benefit, suggesting that different molecular mechanisms may be at play in different people, according to Judith James, a rheumatologist at the Oklahoma Medical Research Foundation.
Luckily, technological advances are enabling researchers to dissect differences—among patients and between diseases—at the genetic level, illuminating patterns that could explain past failures and point the way to future successes. For example, attempts to treat alopecia areata, an autoimmune assault on hair follicles that causes large clumps of hair to fall out, have typically repurposed drugs from the seemingly related skin conditions psoriasis and atopic dermatitis. None of them have worked. When Angela Christiano, a geneticist at Columbia University who has alopecia, completed a study on the genetic basis of the disorder, she suddenly realized why. “You can read it like a road map,” she says. “There’s a reason they failed: we don’t share any genetic pathways with either of those two diseases.” Christiano has learned the illness has more in common with rheumatoid arthritis, celiac disease and type 1 diabetes than it does with skin ailments. “I think it is a great example of how genetics can completely realign your thinking,” Christiano says.
One of the genes she identified, ULBP3, acts as a distress signal that damaged cells use to tell killer T cells to take them out. Normally the gene turns on only if a cell is cancerous, infected or dying. But in hair follicles affected by alopecia, the gene is stuck in the on position, constantly signaling for the cells’ own demise. That signal involves the production of a type of cytokine called a Janus kinase, or JAK. Christiano showed that a class of drugs known as JAK inhibitors, often used to disrupt autoimmune signaling in rheumatoid arthritis, could thwart the killer T cells’ attack on hair follicles. Within a few months of treatment, patients who were once bald regrew full heads of hair. When Christiano was diagnosed with alopecia early in her career, no one could tell her whether she would get better or worse, and the only treatments available involved injecting steroids into her scalp. Now her work has led to multiple advanced trials of JAK inhibitors for the illness.
Six JAK inhibitors are already approved for other autoimmune and inflammatory diseases, and numerous others are in the pipeline. Still, there are no guaranteed breakthroughs, and even the latest targeted treatments could have off-target effects. For example, in February 2021 the U.S. Food and Drug Administration warned of an increased risk of heart-related problems and cancer associated with a JAK inhibitor being used to treat rheumatoid arthritis.
“That’s the reality of autoimmune disease therapy right now, so I always say it’s worth searching for the lesser of all the evils,” says Aimee Payne, a dermatologist at the University of Pennsylvania who is developing gene therapy for a rare autoimmune skin disorder called pemphigus vulgaris. People with this disease have autoantibodies that attack a protein called desmoglein-3 (DSG3), which normally glues skin cells together. When that protein is destroyed, it causes painful blisters to form all over the body, sometimes sending patients into burn units to be treated for life-threatening infections.
Payne has devised a targeted treatment, tested in mice, that could eliminate the specific population of B cells producing these autoantibodies but leave other B cells alone. The immune system has billions of B cells, which come in many different kinds. The vast majority of them produce antibodies needed to fight viruses and bacteria. Fortunately, the anti-DSG3 B cells are easy to find because they have a highly distinctive marker—basically a version of the anti-DSG3 autoantibody stuck on their surfaces. “In some ways, these B cells are terrible criminals because they’re declaring what they’re going to attack before they ever attack it,” Payne says.
To get rid of these disease-causing cells, Payne used a technique invented by her colleague Michael Milone that has successfully eliminated malignant B cells in certain blood cancers. The strategy, called CAR T cell therapy, uses genetically engineered killer T cells that have a kind of homing beacon known as a chimeric antigen receptor (CAR). It steers the T cells to other specific cell types in a search-and-destroy mission. In adapting the technique to treat autoimmune disease, the team gave T cells a beacon derived from bits of the anti-DSG3 autoantibody, which leads the killers straight to anti-DSG3 B cells.
When the researchers infused the engineered T cells into a mouse model of pemphigus vulgaris, the blisters disappeared. Payne founded a biotech start-up to move the treatment forward to clinical trials, which are currently ongoing. Because the treatment is made of living cells that can replicate and remember their target, she thinks a single infusion could last decades. “My dream is the one-and-done,” she says. “A precision cure of disease.”
Other researchers are using a related technique to tip the balance on the other side of the autoimmunity equation. Rather than mobilizing killer T cells, they are amplifying the calming power of regulatory T cells to suppress the overactive immune system. Still in early stages, the approach has shown promise in animal models of ulcerative colitis, multiple sclerosis and rheumatoid arthritis.
Beyond Drugs
Although pharmaceuticals make up the bulk of the growing armamentarium against autoimmune diseases, some of the most intriguing additions explore alternative ways to restore balance in the body.
Many of our basic bodily functions—heart rate, blood pressure, digestion, respiratory rate and sexual arousal—are governed by two opposing forces. The sympathetic nervous system initiates the energetic “fight or flight” response, whereas the parasympathetic nervous system halts this activity and prepares the body to “rest and digest.” Our ability to shift from one state to the other depends largely on the vagus nerve, a bundle of 100,000 nerve fibers that runs from the brain stem down to the diaphragm before shooting out its tendrils to the heart, gut and other organs. Some research suggests this influential piece of anatomy starts to malfunction before people develop autoimmune diseases. “It would be like the brake failing on your car when you are going down the mountain,” says Kevin Tracey, a neurosurgeon at the Feinstein Institutes for Medical Research, based in Manhasset, N.Y.
Tracey has shown that stimulating the vagus nerve with tiny jolts of electricity can reset the nervous system and actually helps to calm overactive immune cells. He and other researchers have traced the way signals from a small electrical device implanted in the neck travel down the vagus nerve all the way to the spleen, where they shut down the production of TNF and other inflammatory molecules. Initial clinical trials suggest a small zap to the vagus nerve could reduce the severity of rheumatoid arthritis and Crohn’s disease, even when delivered through a less invasive device that stimulates a branch of the vagus near the ear when it is pressed against the skin. A company Tracey co-founded recently launched a multicenter randomized, controlled trial of the implantable stimulator. (Similar vagus nerve stimulators are already approved by the FDA for treating epilepsy and depression.)
Another unconventional method looks to correct immune system imbalance with a decidedly lower-tech intervention: fecal transplants. The idea of transplanting fecal matter from one person to another originated in ancient Chinese medicine, when a stool slurry called “yellow soup” was used to treat severe food poisoning and diarrhea. In modern times, fecal transplants have become an accepted therapy for a dangerous gut infection caused by Clostridium difficile, or C. diff.
A few years ago gastroenterologist Jessica Allegretti and other researchers noticed that in patients with C. diff problems, a fecal transplant not only resolved the infection but also helped concurrent cases of inflammatory bowel disease (IBD). The severe digestive distress of IBD stems from inflammation, and some scientists think an autoimmune reaction is involved, although the notion is a matter of some debate. Still, the results “got the community excited about this potential,” says Allegretti, who directs the fecal microbiota transplant program at Brigham and Women’s Hospital in Boston. Plus, the finding makes sense. There are distinct differences in the populations of gut microorganisms—known as the microbiome—between nonaffected people and those with ulcerative colitis, a subtype of IBD that does appear to involve autoimmunity. These changes most likely reflect an imbalanced state where pro-inflammatory microbes dominate over anti-inflammatory ones.
Four randomized clinical trials have tested fecal transplants in ulcerative colitis. In all, about a third of patients went into remission, rates similar to those seen with immunosuppressive medications. More studies and clinical trials of the transplants are underway for rheumatoid arthritis, lupus, multiple sclerosis and alopecia areata, further testing the limits of this odd healing modality.
Out of the Mainstream
Fecal transplants are not FDA-approved for any medical condition, so don’t expect them to emerge as autoimmune treatments anytime soon. Allegretti says the slow track is the right one. “I think there’s a misconception that because this is ‘natural’ it is somehow safer than regular drugs, and I don’t think that’s true at all,” she says. “We are only just starting to understand the long-term consequences of these therapies, and I think they deserve the respect of being studied appropriately in the way we study all drugs.” The scientific literature is littered with accounts of home fecal transplants gone wrong, such as the case of a man with ulcerative colitis who attempted the procedure with stool from his infant son and his wife and landed in the hospital with a cytomegalovirus infection.
Yet many people with autoimmune disorders, frustrated with the lack of conventional therapies and impatient with doctors who offer little help and less hope, are willing to take their health into their own hands, using approaches within and outside mainstream medicine to manage their autoimmune symptoms. Joe Person, for instance, is a 37-year-old based in Washington, D.C., who has lupus. When he was diagnosed in his senior year of college, he drafted a decision tree of the possible tests, complications, outcomes and medications. At his first appointment with his rheumatologist, he demanded to be put on the antimalarial drug hydroxychloroquine, which has helped ease lupus symptoms and is the basis of early treatment and prevention trials.
Fifteen years later Person credits that early action with keeping the illness at bay, although he does have physical fatigue and feels his brain works slowly at times. “The takeaway I’ve gotten from rheumatologists is just sit there and wait for us to invent the pharmaceutical that works,” Person says. “Unfortunately, sitting around and doing nothing is antithetical to every survival instinct in my body. I want to do as much as I can proactively, if nothing else to just feel like I’m exploring all options to stay ahead of this.” He went into remission after switching to a raw vegan diet, only to have his disease flare when he developed an allergy to raw vegetables. And he installed a tanning bed in his basement to test out a theory that certain wavelengths of ultraviolet light can relieve lupus symptoms, although he has yet to see any positive results.
Some scientists are open to alternatives. Tracey says strategies such as acupuncture could, in theory, reduce stress and inflammation by activating certain branches of the nervous system, although he adds that such approaches sometimes make it “hard to prove cause and effect.” To stimulate a healthy gut microbiome without an illegal stool infusion, Allegretti recommends trying fiber-rich foods and plant-based diets. And James advises people with autoimmune problems to avoid smoking and get plenty of sleep, which help the body reduce inflammation: basically “all those things your mother told you,” she says.
For Magdalene Quintero and her sister, their lives feel shaped by their illnesses. Her sister takes as many as 19 medications to manage her lupus symptoms. Quintero needs to take only two drugs right now and has spent the past few years working under the mentorship of James at the Oklahoma medical foundation, first in the lab and now in the clinic, trying to improve treatments for the disease. She remembers how her great aunt, who also had lupus, spent the last years of her life in a wheelchair, her bones ravaged by steroids. “Back then they didn’t have the treatments we have,” she says. Quintero is now trying to get into a medical school or a physician assistant program, determined to put herself in a position to alter the course of the disease that has tormented her family.



