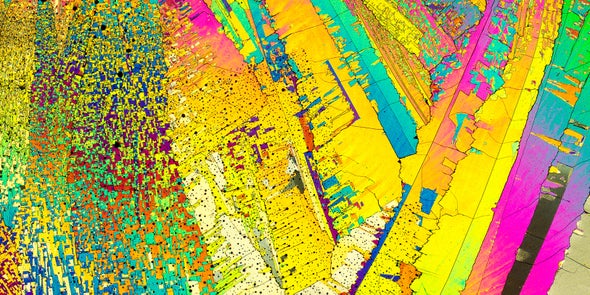
Photographer Justin Zoll shoots stunning landscapes—without setting foot outside. Instead he peers through a secondhand Olympus BH2 microscope into a hidden world of psychedelic crystal vistas.
Zoll has more than 15 years of photography experience, mostly capturing weddings and concerts. A friend introduced him to the world of micrography around six years ago. “I got hooked right away,” Zoll recalls. He fell in love with the trial-and-error process of combining solvents and salts, creating aesthetically pleasing compositions too small to be seen with the naked eye. When the pandemic struck and event venues shut down, he suddenly found himself with ample time to experiment in his makeshift bedroom lab. He ordered a plethora of powdered substances from Amazon and busted out his hot plate.
The vibrant colors in Zoll’s photographs are the product of each crystal’s structure—not added by a computer. “If you look through the microscope, this is pretty much exactly what you see,” he says, though he does take multiple shots of each slide to create a panoramic effect.
Zoll does not have a background in chemistry or physics, but he harbors a deep appreciation for the beauty of science. “Simple math creates similar structures, from the cosmic down to what I’m looking at in my bedroom under a microscope,” he says. “I think that’s really cool. And I think it’s important for us to share that perspective.”
Caffeine is water-soluble and must be dissolved in order to crystallize. But water has high surface tension. To spread the mixture out, Zoll gently taps it with the tip of a pipette dipped in a surfactant—dish soap in this case.
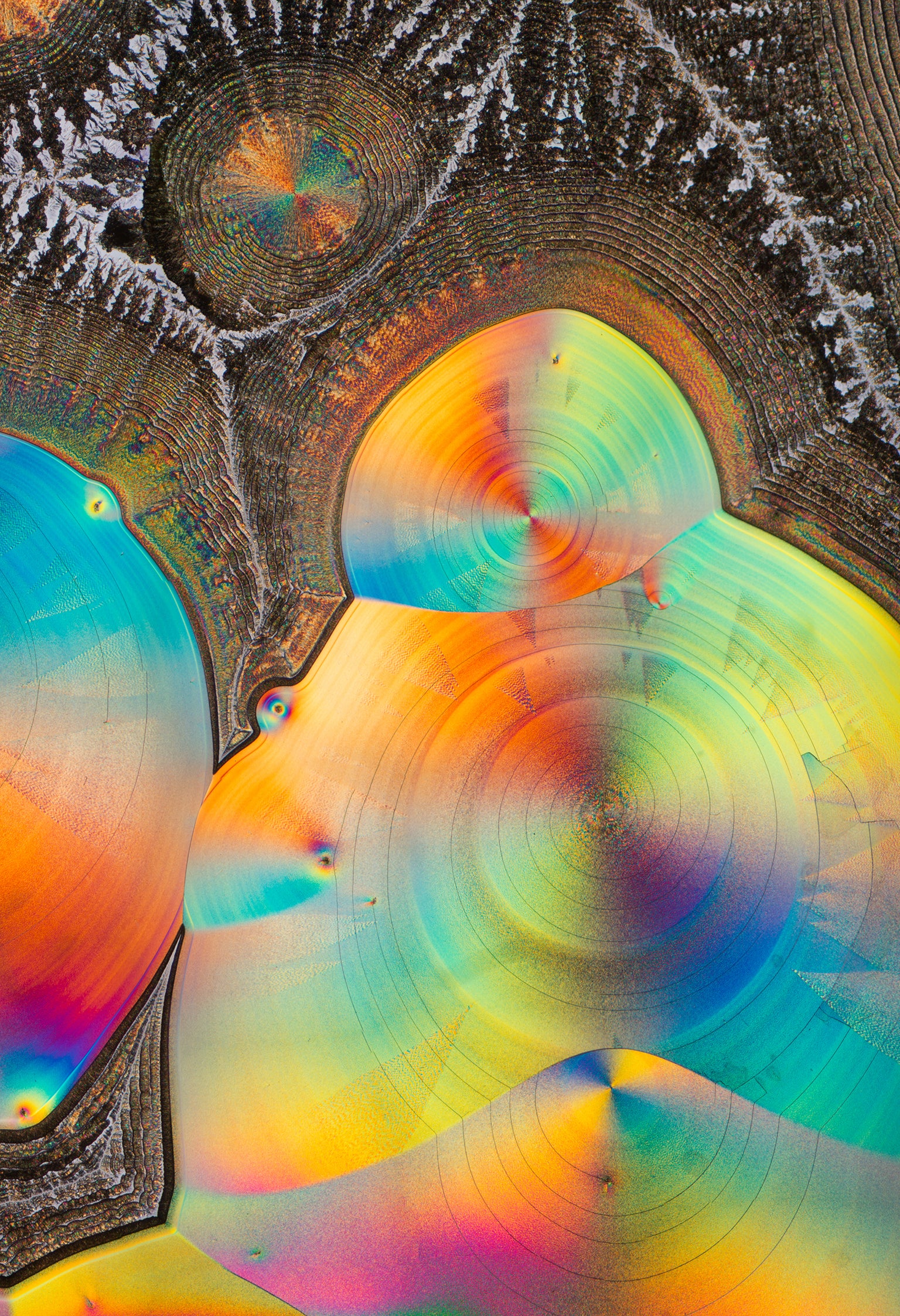
Ascorbic acid, or vitamin C, was one of the first materials Zoll photographed. Depending on its concentration in solution, it can form an astounding variety of crystal shapes, including these spiral structures.

When combining the amino acids L-glutamate and beta-alanine, Zoll typically dissolves the mixture in high-proof vodka and dries it on a hot plate. The aquamarine and gold colors on display are relatively rare for amino acid crystals and are dependent on their structure.
The blue-green background of this image is formed by a layer of sulfamic acid crystals, and the starbursts on top are crystallized synthetic vanillin, an artificial vanilla flavoring. If the vanillin crystals continue to grow, Zoll says, they take over the slide within seconds.
Naphthalene, the main ingredient in a type of mothballs is commonly used to repel insects (and sometimes opossums). Thinly shaved, melted and backlit by polarized light, it looks like a radiant mountain of confetti.
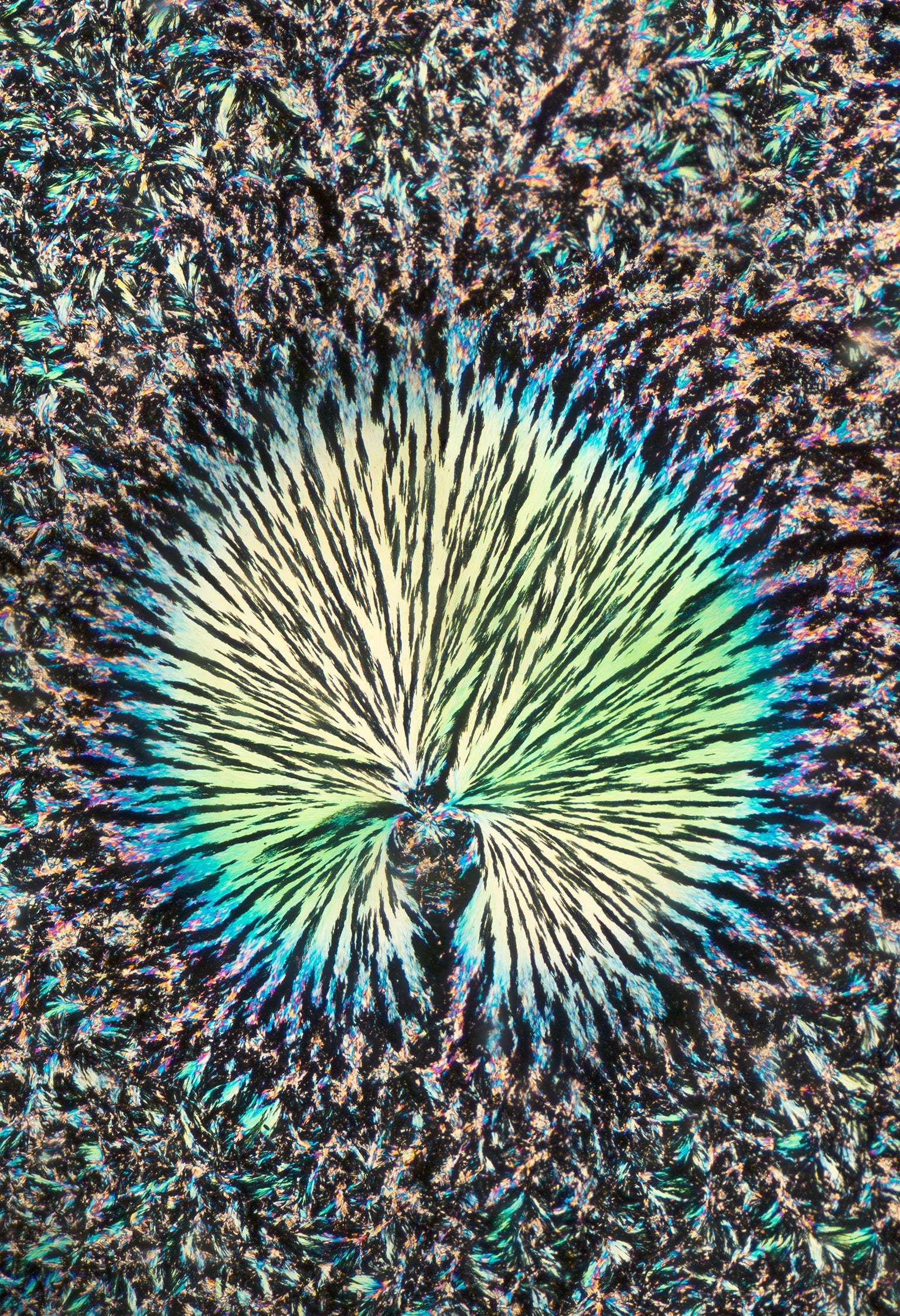
Menthol melts very close to human body temperature—around 98 degrees Fahrenheit. Zoll says he could likely melt a menthol crystal by holding it in his palm and squeezing. It recrystallizes very quickly as well, however. Once the menthol has melted, he has only seconds to snap a photograph.

Sometimes beta-alanine and L-glutamate form thicker, more textured crystals such as the ones pictured here. The result is a range of dramatically lit peaks and valleys in dark red. Zoll loves working with these chemicals for their versatility.
These monk fruit sweetener crystals took about a month to form. Monk fruit contains a compound called mogroside that is used for such sweeteners. Despite tasting sweet, mogroside has a much more complex chemical structure than regular table sugar and looks wildly different under a microscope.
Coenzyme Q10 is a substance that occurs naturally in the human liver, kidneys and heart. The band in this image was likely caused by a disruption during the crystallization process, such as when someone gently blows over a slide.
A version of this article with the title “Science in Images” was adapted for inclusion in the June 2022 issue of Scientific American.